The clinical progress of mRNA vaccines and immunotherapies
来源:Nature Biotechnology | 发布时间:2022-06-02
Abstract
The emergency use authorizations (EUAs) of two mRNA-based severe acute respiratory syndrome coronavirus (SARS-CoV)-2 vaccines approximately 11 months after publication of the viral sequence highlights the transformative potential of this nucleic acid technology. Most clinical applications of mRNA to date have focused on vaccines for infectious disease and cancer for which low doses, low protein expression and local delivery can be effective because of the inherent immunostimulatory properties of some mRNA species and formulations. In addition, work on mRNA-encoded protein or cellular immunotherapies has also begun, for which minimal immune stimulation, high protein expression in target cells and tissues, and the need for repeated administration have led to additional manufacturing and formulation challenges for clinical translation. Building on this momentum, the past year has seen clinical progress with second-generation coronavirus disease 2019 (COVID-19) vaccines, Omicron-specific boosters and vaccines against seasonal influenza, Epstein–Barr virus, human immunodeficiency virus (HIV) and cancer. Here we review the clinical progress of mRNA therapy as well as provide an overview and future outlook of the transformative technology behind these mRNA-based drugs.
Main
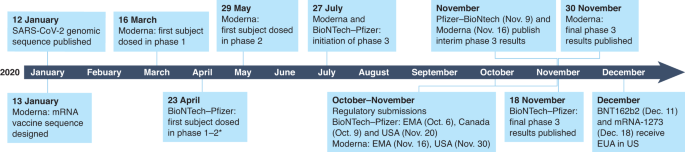
The medical promise of mRNA has been finally realized with the full approval of two rapid-response mRNA vaccines against COVID-19: Comirnity (BNT162b2) and Spikevax (mRNA-1273). Moderna’s mRNA-1273, one of several mRNA vaccines directed against the SARS-CoV-2 spike (S) protein, was first administered to human volunteers on 16 March 2020, within weeks of the virus sequence being published on 11 January 2020 (refs. 1,2). This remarkable achievement was facilitated by almost a decade’s worth of clinical experience with mRNA vaccines for infectious disease and cancer (summarized in Fig. 1).
The concept of using mRNA to encode proteins for either vaccination or protein replacement received its first in vivo validation in 1990, when Wolff et al. demonstrated the production of a target protein after intramuscular (i.m.) injection in mice3. It took several decades, however, before the promise of this technology was clinically validated, a delay due, in part, to technical difficulties with mRNA stability and delivery and an interim shift in research priorities, funding efforts and industry focus to DNA vaccines during the 2000s4. In the meantime, the potential advantages of mRNA as a vaccine moiety (ease and speed of design and testing, inherent immunogenicity, rapid scale up and manufacture5, and negligible risk of insertional mutagenesis6,7) meant that a small number of dedicated academics continued to work on this single-stranded nucleic acid.
One particularly important advantage of mRNA technology arises from its biological role as a template for protein translation. Whereas conventional vaccine technology relies on bulk production of a vaccine using mammalian cells in a bioreactor or chicken eggs, mRNA vaccines turn into the final product only once inside a patient’s cells. In effect, mRNA uses the human body as its own vaccine-production facility, with several accompanying advantages.
First, it allows human post-translational modification (PTM) of protein products with the potential for less immunogenicity and full functionality. Second, multimeric proteins that are not amenable to production in a bioreactor can be translated, folded and assembled correctly in the patient’s cells8 (for example, Moderna has designed a cocktail of five mRNA species for an investigational cytomegalovirus (CMV) vaccine (mRNA-1647) that produces a pentameric protein9,10). And third, mRNA therapy can produce transmembrane and intracellular proteins and traffic them to their appropriate site in the cellular environment.
Compared with viral vector-based modalities, mRNA also has a reduced potential for either pre-existing antibodies against the vector (which limit the patient pool eligible for treatment) or the generation of post-dosing antibodies, which decrease the efficacy of subsequent doses11. Although the generation of anti-vector antibodies for RNA nanoformulations has been observed in preclinical models, methods have been developed to eliminate these responses, enabling repeat dosing without reduced efficacy12,13,14. There are also examples of successful repeat human dosing15 and repeated human dosing with related short interfering RNA nanoformulations16.
Similar to other drugs, the dose of an mRNA can easily be titrated up or down, with a longer or shorter interval, depending on an individual patient’s need, weight and disease state. In addition, the duration of action is intrinsically limited, reducing the likelihood of irreversible side effects and enabling treatment of acute indications7,8; as mRNA degradation is regulated by normal cellular processes, in vivo half-life can be regulated through modifications to the molecule and the delivery methods17,18,19,20.
In the following Review, we provide a broad overview of the clinical landscape of mRNA medicines. We give particular emphasis to technological innovations in manufacturing and formulation that have turned this approach from a vision into approved vaccines, the lessons learned so far from clinical trials and the challenges that we envision for future research, including the prospects of other modalities, such as mRNA-encoded protein and cellular immunotherapies. Because of space constraints, we refer readers to several recent reviews that cover mRNA-transfected dendritic cells (DCs)10,21 or self-replicating mRNA derived from viruses7,9,10, which are not covered here in detail.
mRNA as a medical product
mRNA medicines fall into three basic categories: preventative vaccines, therapeutic vaccines and protein-encoding therapies. Although each application has its own unique set of challenges, one challenge common to all is the requirement for intracellular delivery of the mRNA moiety to target cells while preserving mRNA stability. RNA is intrinsically an unstable molecule, and much of the early work on turning the concept of mRNA medicines into a reality focused on stabilization. Various techniques have been used for this, including optimizing the 5′ cap structure and the 3′ poly(A) tail length as well as regulatory elements within the 5′ and 3′ untranslated regions22 (the reader is referred elsewhere for a detailed discussion of these techniques)23,24,25.
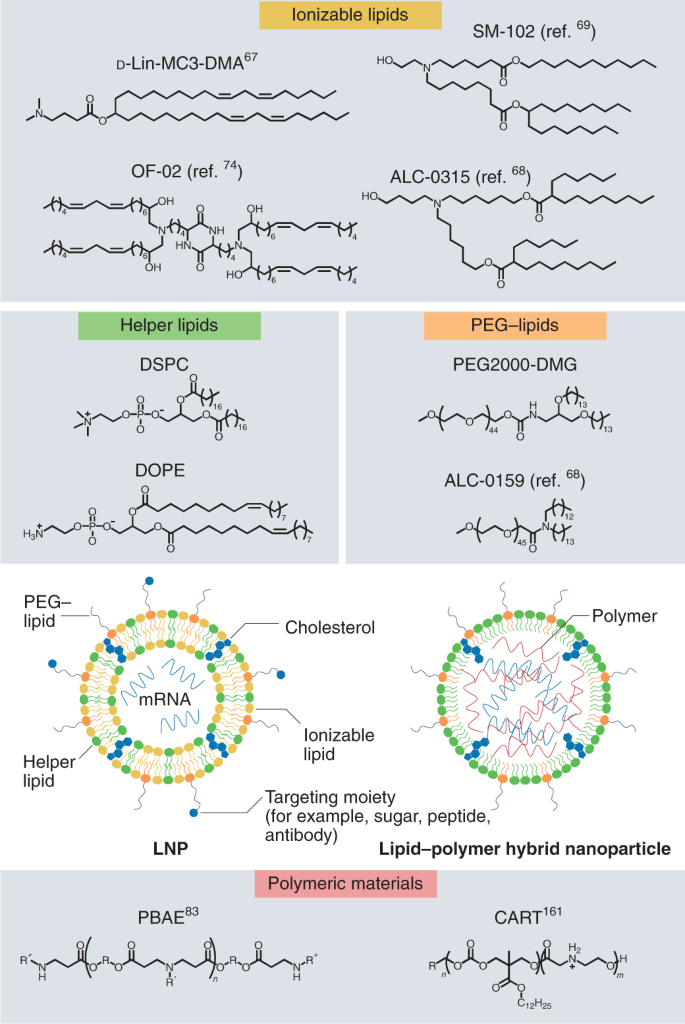
In addition to these advances made in improving mRNA stability, effective in vivo mRNA medicines also required efficient intracellular delivery. A decade’s worth of experimentation, which started with naked mRNA and then explored the condensation of mRNA into nanoformulations, has converged toward an increased focus on lipid formulations to achieve delivery. A typical lipid nanoparticle (LNP) formulation is composed of (1) an ionizable or cationic lipid to interact with the polyanionic RNA, (2) a helper phospholipid (for example, 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine and 1,2-distearoyl-sn-glycero-3-phosphocholine (DSPC)) that resemble the lipids in the cell membrane and support the bilayer structure), (3) a cholesterol analog to adjust the fluidity of the lipid bilayer and (4) a polyethylene glycol (PEG)–lipid to improve colloidal stability and decrease opsonization (Fig. 2).
Although substantially less advanced clinically than LNPs, polymeric nanoparticles (PNPs) have also shown promise as delivery systems. These formulations are generally composed of a biodegradable, amine-containing polymer that can self-assemble with RNA. Depending on the application, PNPs may also be formulated with helper phospholipids, cholesterol and PEG–lipid (Fig. 2). Both LNPs and PNPs may be further modified with specific ligands to facilitate cell-specific targeting. The specific compositions of non-viral vector formulations in development greatly vary and can have substantial effects on the efficiency of intracellular delivery and the cell types targeted by the nanoparticle–mRNA complex as well as immunogenicity of the administered mRNA medicine.
Eliciting appropriate immunogenicity when desirable (vaccines) or to elude it for other indications (mRNA protein-replacement therapy) is an important aspect to consider when manufacturing and formulating mRNA medicines. RNA, as the genetic material of RNA viruses or a byproduct of the replication of DNA viruses, can be a powerful stimulus to the innate immune system. Microbial RNA has a number of structural and sequence characteristics that distinguish it from self RNA that can be recognized by pattern-recognition receptors in host cells. Two systems of pattern-recognition receptors have evolved to orchestrate an appropriate immune response by the production of type I interferons (IFNs) and inflammatory cytokines: the first, the Toll-like receptor (TLR) system, is located in the plasma membrane, endosomes and lysosomes of epithelial and immune cells, including DCs, monocytes and macrophages26; the second, the retinoic acid-inducible gene I (RIG-I)-like receptors, are located in the cytosol of most cells27. TLR3 is activated by double-stranded RNA (dsRNA), whereas TLR7 and TLR8 are activated by single-stranded RNA. RIG-I and melanoma differentiation-associated protein 5 (MDA5) are differentially activated in the cytosol by 5′-triphosphorolyated short (18–19 bp) dsRNA and long (>1,000 bp) dsRNA, respectively27. TLR3 activation leads to the production of type I IFN via the TIR domain-containing adaptor molecule 1 (TICAM-1) pathway, whereas the other TLRs use a MYD88-dependent cascade that leads to a nuclear factor (NF)-κB-dependent or an IFN regulatory transcription factor (IRF)3-dependent production of pro-inflammatory cytokines28. The extent to which these pathways are activated, if at all, by the mRNA product and its delivery vector greatly depends on the application and is a key aspect in the development of mRNA medicines.
The landscape of biomedical uses of mRNA continues to rapidly evolve. Below, we divide our discussion into three areas: direct in vivo administration of mRNA for preventative vaccines against infectious disease (Table 1), therapeutic mRNA vaccines against cancer (Table 2) and mRNA-encoded immune therapies (Table 3). We refer the reader to Supplementary Table 1 for a complete list of all mRNA species in clinical testing at the time of writing. To provide up-to-date information on clinical advances, we have used as sources papers indexed in PubMed, company press releases, postings at https://clinicaltrials.gov/ and US Securities Exchange Commission filings through August 2021.Table 1 Summary of past and ongoing clinical studies with mRNA vaccines for infectious disease, phase 2 or 3 only
Full size tableTable 2 Summary of past and ongoing clinical studies with mRNA vaccines for cancer, phase 2 or 3 only
Full size tableTable 3 Summary of past and ongoing clinical studies with mRNA for protein-replacement applications, phase 2 or 3 only
Vaccines for infectious disease
Following the US Food and Drug Administration (FDA) approvals for COVID-19 vaccines, mRNA is now recognized as a potentially transformative vaccine modality in infectious disease (Table 1). The field eagerly awaits further validation of mRNA vaccines directed against pathogens other than SARS-CoV-2.
The mRNA molecule’s inherent immunostimulatory nature and ability to function as an immunoadjuvant were seen as a key strength for vaccine application22. Turning these characteristics into a safe and efficacious clinical product presents the challenge of balancing immune stimulation with expression of the encoded antigen. Thus far, the most clinically advanced products are non-replicating mRNA vaccines featuring chemically modified and unmodified nucleotide bases. The two approved mRNA products, Pfizer–BioNTech’s BNT162b2 and Moderna’s mRNA-1273, are vaccines with chemically modified uradine bases.
By contrast, the results of unmodified RNA vaccine trials for COVID-19 thus far have been disappointing. Although definitive data remain to be released, CureVac reported that its unmodified CureVac COVID-19 vaccine (CVnCOV) shows only 47% protection against coronavirus infection29. Various explanations have been put forward to account for the lower efficacy of the unmodified CureVac vaccine compared with that of modified vaccines. CureVac has pointed to the changing SARS-CoV-2 variant landscape during the conduct of its trial, which was not the case for BNT162b2 or mRNA-1273; others have noted CureVac’s use of a lower dose (12 µg) versus those of BNT162b2 (30 µg) and of mRNA-1273 (100 µg) (chosen as unmodified RNA is more reactogenic than modified RNA) may have been insufficient to produce an effective neutralizing antibody response; others have hypothesized that translation efficiency of unmodified RNA may be lower, resulting in lower epitope levels30.
Apart from the above non-replicating mRNA vaccines, several groups are also pursuing self-amplifying constructs encoding RNA-dependent RNA polymerases that amplify the delivered RNA and thus increase antigen protein expression13. As of yet, these have only completed early-stage clinical testing. One potential drawback of this last type of vaccine is that any mRNA-delivery technology must contend with the substantially larger mRNA construct sizes associated with self-replicating mRNA vaccines.
Similar to recombinant protein vaccines, all the above mRNA vaccines have the advantage of not producing infectious particles. Thus, concerns associated with live attenuated viral vaccines or replication-competent viral vectors and their potential to revert to a pathogenic form or cause some form of exacerbated disease (as has been observed with a live attenuated respiratory syncytial virus (RSV) vaccine) do not apply. The absence of risk of insertional mutagenesis caused by integration into the recipient’s DNA is another major advantage of mRNA vaccines compared with DNA vaccines or certain viral vectors.
Accelerated discovery and development times
The rapid spread of the SARS-CoV-2 pandemic across the globe highlighted the importance of vaccine technologies capable of rapid deployment for human trials. The speed of mRNA vaccine development was such that the first products had already entered clinical trials before studies in non-human primates confirmed that protective immunity could be achieved either by infection with SARS-CoV-2 (ref. 31) or by a DNA vaccine32.
The unprecedented speed with which mRNA companies pivoted toward producing SARS-CoV-2 vaccine candidates is illustrated by the chronology shown in Fig. 1 (refs. 1,2). The remarkably similar timelines of two independent efforts of Pfizer–BioNTech33 and Moderna34 indicate a trajectory from genetic identification of a pathogen to EUA by regulatory agencies ~11 months or years shorter than the typical vaccine-development timeline.
Other mRNA companies have also been able to leverage their prior expertise in vaccine development in comparably rapid ways. For instance, CureVac announced approval to start a phase 1 study of CVnCOV in June 2020 (NCT04449276)35 and interim results of its phase 2–3 trial (HERALD, NCT04652102) a year later; Arcturus announced interim results of a phase 1 study of ARCT-021 (NCT04480957) on 9 November 2020 (ref. 36); and Sanofi Pasteur–Translate Bio (NCT04798027)37, the Imperial College of London (ISRCTN17072692, Eudract 2020-001646-20) and Yunnan Walvax Biotechnology (ChiCTR2000034112) all took mRNA vaccines to human testing in less than a year from publication of the SARS-CoV-2 sequence.
The COVID-19 pandemic has challenged traditional approaches to vaccine development and created a unique environment to galvanize mRNA vaccine research. One key differentiator was the large injection of funding that companies received from the Biomedical Advanced Research and Development Authority and the Coalition for Epidemic Preparedness Innovations1,38,39,40. The public health emergency served to catapult development efforts into high gear and prompted manufacturers to find ways to reduce the time to clinic (for example, by parallelizing different parts of the serial development process, minimizing pilot studies and conducting minimal product-quality release testing); conversely, extensive validation of the new mRNA technology against established vaccines (as had been done previously in the side-by-side evaluation of mRNA vaccine CV7202 versus an inactivated strain vaccine, Rabipur41) was de-prioritized. For in-depth discussions and comparisons of the various technologies currently established or in development for vaccines, we refer the reader to some excellent reviews39,42,43,44.
Manufacturing and scale up
Many of the advantages of mRNA (and some types of DNA) vaccines relate to the speed and flexibility of manufacturing, which is largely based on in vitro processes with chemical constituents. Because mRNA codes for the immunogenic protein of interest and no live virus is required, there is no need for specialized facilities or biosafety laboratories44. In contrast to egg-based vaccines, mRNA vaccines are not limited by egg-production capacity and allow vaccination of individuals with egg allergies39. Production in cell-free systems minimizes the risk of bacterial contaminants and eliminates the need for bioreactor processes39.
All nucleic acid-based vaccines (whether mRNA or DNA) encode the immunogen of interest, but their characteristics are independent of that immunogen. The manufacturing of different vaccines with the mRNA platform relies on the same chemical components, which means that, once an investment has been made in the platform, it can readily be adapted to new pathogens as they are identified44. This is a particularly attractive feature in the context of preparedness for emerging epidemics or seasonal vaccines.
Furthermore, the same manufacturing processes can be used for vaccines and other mRNA-based medicines, providing efficiency and flexibility. In view of the emergence of new strains of SARS-CoV-2 while the rollout of the first-generation vaccines is underway, this flexibility to switch out the coding mRNA in the same delivery vehicle is especially useful. Although there is no consensus about the cost of manufacturing, the technology is expected to be more cost-effective than older methods43.
Rapid antigen-specific sequence optimization
Another advantage of mRNA technology is the ability to design and redesign the antigen based on introducing changes in nucleic acids, which is a relatively straightforward process compared to the bioengineering of distinct proteins or peptides. For SARS-CoV-2, this has mainly taken the form of introducing prolines to stabilize the immunogenic S protein into the prefusion configuration.
BioNTech has applied this technical flexibility by putting no fewer than five different COVID-19 mRNA vaccine candidates into the clinic (three using nucleoside-modified mRNA, one using uridine-containing mRNA and one using self-amplifying mRNA). The BNT162b1 vaccine candidate uses nucleoside-modified mRNA to encode the SARS-CoV-2 S protein receptor-binding domain modified by the addition of a T4 fibritin-derived foldon trimerization domain to increase its immunogenicity45. BNT162b2, the final selected candidate, encodes full-length S protein modified by two proline substitutions to lock it in the prefusion conformation46. The other COVID-19 mRNA vaccines from CureVac29,47, Moderna48 and Translate Bio49 also use the S protein with various modifications.
Several of the vaccines currently in development have gone through at least one iterative optimization step, which is a feature of mRNA product development. For instance, mRNA-1777, which targets RSV, was tested in a phase 1 trial, and interim data showed humoral immune responses as measured by neutralizing antibody levels after a single dose50. However, development of this drug has been paused in favor of mRNA-1172, which was shown to be more potent than mRNA-1777 in animal models38. Similarly, development of the Zika virus vaccine candidate mRNA-1325 (NCT03014089) was halted in favor of mRNA-1893 (ref. 38), which uses a different sequence and was reported to be 20-fold more potent than mRNA-1325 in non-human primate studies. As of February 2020, 90 participants, both flavivirus seropositive and seronegative, have been administered mRNA-1893 or placebo in a dosing regimen of two doses 1 month apart, at doses of 10, 30 and 100 µg (NCT04064905)38,51.
Encoding multiple proteins and/or protein subunits
For SARS-CoV-2, the S protein immunogen is a homotrimer52, and thus only a single mRNA sequence needs to be introduced. For pathogens for which the main immunogen is composed of multiple subunits, the challenges for producing recombinant protein subunits and successfully reconstituting them with the correct stoichiometry into a full protein can readily be imagined. By contrast, mRNA lends itself easily to this application. The separate subunits can either be coded in a single long mRNA or as separate mRNA strands. For instance, mRNA-1647, a vaccine targeting CMV, contains six mRNA species, five of which encode five different proteins that combine to form a pentameric protein, with the sixth encoding the CMV glycoprotein B (gB) protein38. In a related approach, a single vaccine can target two different pathogens, as is the case for mRNA-1653, which combines two mRNA species, targeting the F protein of human metapneumovirus (hMPV) and parainfluenza virus type 3 (PIV3)38.
Modulating mRNA immunogenicity
Apart from iterative optimization of sequence to optimize the immunogenicity of antigens, innate immunogenicity of the full mRNA transcript itself and other RNA products produced during its manufacture can also be exploited to boost immune responses to mRNA vaccines. The innate potential immunogenicity of RNA may be advantageous in vaccinations because it can activate immune response pathways, such as the TLR system, that lead to DC maturation and subsequently robust B and T cell immune responses53,54,55. As mentioned previously, CureVac’s CVnCoV features unmodified mRNA47. This RNA-driven immunostimulation, however, can also be detrimental, leading to clinical side effects as well as reduced expression of the protein of interest. Activation of RNA-dependent protein kinase R (PKR), for instance, has been implicated in translational inhibition56.
In recent years, progress has been made in our understanding of how to modulate in vitro transcribed mRNA immunogenicity. One of the main methods for modulating mRNA immunogenicity has been substitution of unmodified nucleotides with chemically modified versions. Work by Kariko, Weissman and colleagues reported that certain nucleoside modifications, such as pseudouridine and 5-methylcytidine, significantly reduce TLR signaling and PKR activation, leading to increased levels of protein expression in mice56,57. Notably, both approved COVID-19 mRNA vaccines from BioNTech and Moderna (BNT162b2 and mRNA-1273) feature complete substitution of uridine with N1-methyl pseudouridine2,58. When compared with mRNA with modified nucleosides, however, others have subsequently demonstrated that incorporation of unmodified nucleosides actually leads to higher levels of protein expression in HeLa cells and similar levels of expression in the liver of mice59. We hypothesize that improvements in mRNA purification and the removal of RNA contaminants may in part explain these differences from earlier work. Removal of dsRNA by column purification (high pressure liquid chromatography (HPLC) or fast protein liquid chromatography) and more recently by less-expensive filter-binding technology leads to substantially improved translation efficiencies60,61. Researchers at CureVac reported that HPLC-purified, sequence-optimized, unmodified nucleoside mRNA is not immunogenic and produced higher levels of protein expression in mice than chemically modified nucleoside mRNA18. Recently, scientists at Genentech have reported that interleukin (IL)-1β and IL-1 receptor agonist (IL-1RA) are key regulators that control systemic responses to mRNA, suggesting that differences between these regulatory elements in mice, primates and humans may explain the observed differences in reactogenicity of uridine-modified and unmodified mRNA in vivo in these species62.
Nanoformulations for mRNA delivery
Although early efforts for mRNA vaccine delivery focused on naked mRNA or the use of protamine, recent trends in mRNA vaccine development have converged on LNPs for delivery of mRNA. An early rabies vaccine (CV7201) was formulated with protamine, but development was discontinued because the level of immunogenicity seemed critically dependent on the method of vaccination, with only a needle-free system providing the desired immune response after intradermal (i.d.) administration (NCT02241135)63; the development of this product was halted in favor of an LNP-formulated vaccine candidate, CV7202 (NCT03713086)64. Elsewhere, a naked mRNA agent (iHIVARNA-01), which combines TriMix (a mix of three mRNA species encoding constitutively activated TLR4, CD40 ligand and CD70, all of which are immunostimulatory molecules) and an HIV immunogen, has been evaluated for safety and efficacy in patients with HIV after three intranodal (i.n.) injections (NCT02413645); however, this study has since been discontinued due to lack of immunogenicity (NCT02888756).
Most mRNA medicines in the clinic now use LNPs for delivery. The first RNA-based oligonucleotide drug approved by the FDA (patisiran, a short interfering RNA drug for the treatment of the polyneuropathy of hereditary transthyretin (TTR)-mediated amyloidosis) is an LNP formulation comprising an ionizable lipid, D-Lin-MC3-DMA (MC3), together with DSPC, cholesterol and 1,2-dimyristoyl-rac-glycero-3-methoxypolyethylene glycol-2000 (PEG2000-DMG)65. The BioNTech COVID-19 vaccine BNT162b2 is formulated using ((4-hydroxybutyl)azanediyl)bis(hexane-6,1-diyl)bis(2-hexyldecanoate) (ALC-0315), 2-((PEG)-2000)-N,N-ditetradecylacetamide (ALC-0159), DSPC and cholesterol66. It appears that the identification, testing and production of the appropriate lipid formulation may have been important in determining the speed of entry into clinical trials. For instance, mRNA-1273 uses the same LNP as mRNA-1647 (CMV virus vaccine) and mRNA-1653 (hMPV–PIV3 virus vaccine), for which some clinical and regulatory precedent had been established (Table 1)67.
The composition of an LNP formulation can substantially affect intracellular delivery efficiency, determine cell specificity of delivery and modulate immunogenicity. Although all lipid components are important for LNP function, the ionizable lipid component of LNPs has received much attention given its key role in multiple aspects of RNA delivery, including particle formation, cellular uptake and endosomal escape10,24. The structural diversity of ionizable lipids found within LNP formulations is vast, and, to facilitate rapid synthesis and evaluation of ionizable lipids, combinatorial, high-throughput methods for synthesizing large libraries of new lipids and evaluating them in vivo have been developed68,69,70,71. The list of potent ionizable lipids capable of delivering mRNA in vivo continues to expand, with advances in both the potency of delivery vehicles72 and tolerability through the introduction of biodegradable linkages73,74. Although the recent trend in lipid development has focused on the incorporation of hydrolysable bonds to facilitate clearance, these degradable bonds may affect formulation stability, which continues to be a challenge for LNP formulations. Advances in lyophilization of mRNA LNPs seem likely to improve formulation stability, but, for low-dose applications (for example, vaccines), hydrolytically resistant lipids may prove advantageous.
In addition to systemically delivered RNA nanoparticles, other modes of RNA application offer the potential to provide therapy to the nervous system75, eye76,77, heart78,79 and lung80,81,82,83. Of particular note, nanoformulations based on both biodegradable polymers81 and oligo polymers82 as well as lipids83 have been developed to facilitate delivery to the lung epithelium by nebulization. For instance, patients with cystic fibrosis have been dosed repeatedly with MRT5005, a nebulized formulation of an LNP-formulated mRNA coding for the cystic fibrosis transmembrane regulator protein (NCT03375047).
Storage and shipping
An important aspect related to formulation is storage and shipping conditions. The challenge of maintaining cold-chain shipping and storage for vaccines was highlighted during the Ebola epidemic of 2014–2016, when an investigational vaccine based on an attenuated recombinant vesicular stomatitis virus (approved in 2019 as Ervebo) had to be stored at −80 °C to −60 °C, which was not always practicable in the regions of Africa where the vaccine was most needed. COVID-19 affects all continents; from a global emergency preparedness perspective, it is imperative that millions of doses could be shipped worldwide and across a range of extreme temperatures to countries with widely differing standards of health care infrastructure39.
CureVac’s CVnCoV has been reported to be stable and within defined specifications for at least 3 months when stored at a standard refrigerator temperature of +5 °C (+41 °F) and up to 24 h as ready-to-use vaccine when stored at room temperature84. Moderna’s mRNA-1273 remains stable at −20 °C for up to 6 months, at refrigerated conditions for up to 30 d and at room temperature for up to 12 h85. By contrast, BioNTech’s vaccine originally needed to be stored at −70 °C, and BioNTech’s collaborator Pfizer had developed specific shipping boxes containing dry ice to facilitate the logistics of distribution. Subsequently, the sponsor submitted additional information supporting up to 2 weeks of storage at standard freezer temperatures86,87.
The first-in-human trial, which usually involves only a limited number of participants and clinical trial sites, is sometimes performed with a less-than-optimal formulation, with the development of improved storage conditions proceeding in parallel with the clinical program. For instance, Moderna’s mRNA-1647, a CMV vaccine candidate, was provided as a frozen liquid formulation for the phase 1 study but as a lyophilized formulation, stable at refrigerated temperatures for 18 months, for the phase 2 study40.
The importance of these considerations of shipping, storage and stability is highlighted by the case of mRNA-1443, which targets the phosphoprotein 65 T antigen of CMV. This was evaluated in the same trial as mRNA-1647, but, in August 2018, the clinical material for mRNA-1443 failed to meet internal specifications after 1 year of storage and was subsequently the subject of a clinical hold88,89. Development of this vaccine appears to have paused89.
Turning the formulation into a dry powder form is among the most commonly used methods for shipping and long-term storage of many pharmaceutical products. However, the stresses generated by crystallization and vacuum dehydration during the lyophilization process may decrease the stability of macromolecules or LNPs, inducing the loss of activity90. Adding appropriate cryoprotectants, such as trehalose, sucrose and mannitol, is reported to preserve the stability of LNPs in a formulation-specific manner90,91. Pfizer has initiated a phase 3 study to compare the safety and tolerability of the lyophilized BNT162b2 formulation to those of its frozen liquid BNT162b2 formulation (NCT04816669).
Route of administration
There is no consensus yet as to the best route of administration, although the approved SARS-CoV-2 mRNA vaccines employ i.m. administration. Global rollout of pandemic vaccines is ideally supported by a low-tech route of administration requiring little training of the numerous health care providers called upon to administer the vaccine. BTN162b2, CVnCoV, ARCT-021 and mRNA-1273 are administered via i.m. injection. A group at the Imperial College of London is considering studying their self-amplifying mRNA COVID-19 vaccine after inhalation, similar to what has been done for seasonal influenza92. An inhaled or intranasal vaccine may elicit both cellular and humoral responses that are particularly effective at neutralizing infectious respiratory viruses such as SARS-CoV-2 (refs. 93,94,95); however, studies of intranasal mRNA vaccines have been limited to preclinical animal models96, with further development of LNP carriers likely required to effectively target appropriate cell types in the upper respiratory tract.
In the broader mRNA field, both i.d. and i.m. injections have been used for the evaluation of candidate vaccines, sometimes for the same vaccine within the same study. The two routes of administration can yield divergent results, both in terms of immunogenicity and in terms of tolerability. mRNA-1440 (VAL-506440), which is directed against the hemagglutinin (HA) protein of the H10N8 strain of influenza, was tested in a phase 1 study in healthy volunteers (NCT03076385), using both i.m. (25–400 µg) and i.d. administration (25–50 µg) routes97.
The i.d. route caused more injection-site reactions than the i.m. route and was not pursued, even though, at 25 μg, it appeared to be more immunogenic than the i.m. route. Two of the three participants vaccinated with 400 µg i.m. experienced severe adverse events of headache and erythema, and the safety committee stopped further dosing at this level38,97. As we have seen, the rabies vaccine CV7201 elicited an immune response only when administered via a needle-free system, and this was both for the i.m. and i.d. route (NCT02241135)63. An early HIV vaccine (iHIVARNA-01) was administered i.n., but this seems to have been the only such study98. At the present time, it seems that i.m. injection is the most widely used route of administration of infectious disease mRNA vaccines, identical to the case with protein and DNA vaccines.
Dosing regimens
The ideal dosing regimen, especially for global prevention, is a single dose with 100% seroconversion soon after the dose. But because of the phenomenon of booster immunity, most dosing regimens include at least two shots, typically a few weeks apart. This is also true for the SARS-CoV-2 mRNA vaccines: BNT162b2 is given as two i.m. injections 21 d apart; for CvnCOv and mRNA-1273, a booster shot is given 4 weeks after the prime. BNT162b2 and mRNA-1273 obtained EUA for their respective dosing regimens. However, due to early vaccine shortages and the partial protection observed after a single dose, some advocate immunizing larger populations with a single dose, rather than reserving part of the supply for the second shot99, or to space out the injections longer than studied in the pivotal clinical trials. Data from a small trial in adults older than 80 years have indicated that spacing the two BNT162b doses approximately 3 months rather than 3 weeks apart enhances the peak antibody generation, while the results regarding cellular immunity are less clear100. In parallel, there is a growing public realization that a third (booster) shot is indicated for optimal control. Recent data showing a slight waning of the effectiveness of the vaccine after 6 months101 and the emergence of new strain variants have infused new urgency into this question.
Self-amplifying mRNA vaccines, such as ARC-021 and BNT162c2, are intended to be given as a single dose. More elaborate regimens have also been described. For instance, mRNA-1647, directed against CMV, was administered in a phase 1 study (NCT03382405) to healthy volunteers, who received three doses of 30, 90, 180 or 300 µg mRNA-1647 or placebo at months 0, 2 and 6 (ref. 38). The RSV vaccine mRNA-1345 is being investigated in a phase 1 study (NCT04258719) as a three-dose injection regimen with doses 2 months apart, and this program has now entered phase 3 as a single-dose regimen102.
Despite the similarities in technology and choice of antigen, the SARS-CoV-2 mRNA vaccines in development cover a wide dose range. As is to be expected based on the technology, self-amplifying mRNA vaccines use smaller amounts per dose: the Imperial College self-amplifying mRNA COVID-19 vaccine is being tested in doses between 0.1 and 1 μg. ARCT-021 was tested at doses between 1 and 10 μg as a single dose and a prime–boost regimen; the 7.5-μg dose will be taken forward for further development36. BNT162b2 and mRNA-1273 were successful in preventing approximately 95% of COVID-19 cases at doses of 30 μg and 100 μg103,104,105, respectively. CVnCOV is being tested in a phase 3 trial at a dose of 12 μg (NCT04652102)106. Outside the SARS-CoV-2 field, the dose range is equally broad. Across different vaccines, the dose levels studied have ranged 300-fold, from 1 μg (CV7202)107 to 300 μg (mRNA-1653 and mRNA-1657)38. Dosing amounts and regimens, along with the storage logistics of the mRNA vaccine, have enormous implications for global immunization plans: the most impactful COVID-19 vaccine or vaccines for future pandemic viruses may not be the first to receive EUA but the first to produce millions of doses and deliver them effectively to the point of service.
Role as adjuvant
As discussed above, RNA can have inherent immune-activating properties. As a supplement or alternative to immune stimulation via innate RNA sensing, some groups have added stimulatory molecules to their vaccines to potentiate the immune response to the encoded antigen with varying degrees of success. For instance, CureVac has used CV8102, a noncoding uncapped single-stranded RNA complexed with a cationic peptide carrier to boost the immunogenicity of a rabies vaccine108,109. CureVac’s RNActive vaccine technology platform, the basis for the discontinued CV7201 vaccine mentioned previously, relies on a two-component mRNA vaccine in which naked mRNA is used for antigen expression while the same mRNA complexed with protamine is used as an adjuvant that activates TLR7 and TLR8 signaling53,110,111. Stimulation of TLR signaling pathways then leads to activation of DCs as part of the innate immune response to the protamine complex53. Another product, iHAVARNA-01, which combines DC-activating mRNA species encoding TriMix and an mRNA encoding HIV immunogen (derived from the consensus Gag protein of HIV-1 clade A and a string of CD8+ T cell epitopes)10,98,112,113.
However, the use of adjuvants for mRNA vaccines seems to be an exception; both BNT162b2 and mRNA-1273 rely solely on mRNA–LNP formulations without the use of adjuvants, and most companies developing mRNA vaccines in the clinic follow the adjuvant-free approach. This may be because LNP components themselves stimulate specific elements of the immune system, such as the stimulator of IFN-γ (STING) pathway and the TLR–RIG-I-like receptor (RLR)-independent mediator of innate immune responses114. The ability of nanoformulations to both deliver mRNA to appropriate cellular targets and selectively stimulate the immune system by design is an additional strength of mRNA as a vaccine platform.
Adverse events
By their very nature as preventative, non-therapeutic agents, vaccines against infectious agents are held to a high standard of safety and tolerability. To date, the safety profile of the RNA vaccines discussed in this review is in line with that of protein-based vaccines. Local injection pain and local or systemic inflammatory reactions (fever, malaise) are the most frequently noted adverse events38,63,97 The two COVID-19 mRNA vaccines that have been administered to more than 30,0000 healthy volunteers, including older people, represent the best dataset for evaluation of the side effect profile, but the comparison must bear in mind that there is a threefold difference in the dose level (and thus of both mRNA and lipid administered) between BNT162b2 (30 μg) and mRNA-1273 (100 μg). In addition, the trials have exclusion criteria that eliminate some of the highest-risk participants (for example, prior history of anaphylaxis) and are thus not necessarily representative of the complete population requiring protection. Both in the BNT162b2 and mRNA-1273 phase 3 studies, more than 80% of vaccine recipients reported local adverse events, mainly pain. The systemic events were mainly headache, fatigue, temperature elevation, myalgia and arthralgia103. For mRNA-1273, the frequency and severity of the adverse reactions tended to be more pronounced after the second dose103. It is not clear what the relative contribution of mRNA and LNP was to these adverse events, as the placebo in these phase 3 trials was 0.9% saline, not naked mRNA or empty LNPs.
An increase in the severity of the adverse events after the second dose may reflect increased reactogenicity and was also observed with the much smaller dataset of the phase 2 study of mRNA-1647, a CMV vaccine administered both to CMV-positive and CMV-negative participants. No difference was observed in safety profile between the two patient groups, but there was a trend toward more frequent and slightly more severe adverse events after the second vaccination67.
Even very large trials, such as for the two mRNA COVID-19 vaccines, are limited in their ability to detect very rare but potentially worrisome adverse events. Reports of myocarditis occurring in young males in the days to weeks after vaccination have prompted the FDA Advisory Committee on Immunization Practices to review the benefit and risk of COVID-19 vaccines. According to their calculations, the risk of myocarditis is highest in the young male population (anticipated 39–47 occurrences per million vaccine doses administered in the group aged 12–29 years), but the benefits (prevention of 11,000 cases of COVID, 139 intensive care unit admissions and six deaths) outweigh the risks115.
Therapeutic vaccines for cancer
The recent explosion and success of cancer immunotherapies has fueled interest in the use of mRNA therapies for this application116 (Table 2). For mRNA cancer immunotherapies, one approach is modification of the immune-suppressive tumor microenvironment through the expression of deficient or altered tumor suppressor protein. However, current mRNA-delivery modalities are unlikely to reach every cancer cell in a patient. Instead, there is increasing focus on the use of mRNA as a therapeutic vaccine to train the immune system to seek out and kill cancer cells. Key characteristics of mRNA vaccines that enabled their success as SARS-CoV-2 vaccines and as vaccines for infectious diseases in general, including the ability to rapidly develop and manufacture the mRNA medicine as well as the ability for mRNA to encode whole antigens, make their use as cancer vaccines particularly promising. Furthermore, many patients have tumors that are resistant to current immune-targeting drugs117, creating a new opportunity for mRNA-based approaches.
The development of therapeutic cancer vaccines, regardless of modality, faces a number of challenges that must be addressed for successful clinical translation. Unlike prophylactic vaccines for infectious diseases for which protection against infection is largely, if not entirely, conferred by a robust humoral response, therapeutic cancer vaccines must also ensure that a strong cytotoxic CD8+ T cell response is induced to eradicate cancerous cells. Although prophylactic vaccines for cancers are possible, there are currently only two FDA-approved cancer-related vaccines and both are against viruses known to cause cancer (human papillomavirus (HPV) and hepatitis B virus). Another challenge is the selection of proper antigens that are able to induce highly tumor-specific immune responses, due to the high variability of antigens across different individuals118. The increasing trend toward patient-specific neoantigens aims to address this challenge119,120. Finally, even if an antigen is able to induce a cellular immune response, the suppressive tumor microenvironment could prevent T cell infiltration into tumors and could lead to T cell exhaustion. Therefore, therapeutic vaccines may require administration in combination with another therapy designed to overcome the suppressive microenvironment such as immune checkpoint inhibitors, as has been posited for BNT111, as described below121.
Tumor-associated antigens
Tumor-associated antigens (TAAs) are preferentially expressed on the surface of tumor cells and represent targets for immune killing of tumor cells. Cancer vaccines targeting TAAs involve the production of fixed, off-the-shelf TAAs for a variety of tumors. The most advanced of these, BNT111, is a mix of four melanoma-related antigens (New York esophageal squamous cell carcinoma 1 (NY-ESO-1), tyrosinase, melanoma antigen family A3 (MAGE A3) and transmembrane phosphatase with tensin homology (TPTE)) that is being evaluated in a phase 1–2 trial (Lipo-MERIT, NCT02410733) either as monotherapy or in combination with a checkpoint inhibitor. This vaccine is given as repeated intravenous (i.v.) administrations, starting with a series of eight injections and with the potential for additional monthly injections and has now progressed to phase 2 in combination with cemiplimab for advanced melanoma (NCT04526899).
The immunological effects of BNT111 in the above study have been reported in some detail15. The mRNA sequence for each of the four TAAs was optimized for translation in immature DCs. Each sequence also contains a signal peptide and the tetanus toxoid CD4+ epitopes P2 and P16 as well as the major histocompatibility complex (MHC) class I trafficking domain for enhanced human leukocyte antigen (HLA) presentation and immunogenicity. Activation of lymphoid tissue was shown by an increase in metabolic activity in the spleen, as measured by 18F-fluoro-2-deoxy-2-D-glucose positron emission tomography of the spleen. About 75% of the 50 evaluated patients showed an IFN-γ response against at least one of the four TAAs by enzyme-linked immune absorbent spot assay, indicating induction of an immune response. The antigen-specific T cells were of the OD1+CCR7−DD27+/−D45RA− effector memory phenotype and secreted IFN-γ and tumor necrosis factor upon stimulation. In patients continuing to receive vaccinations, the TAA-specific cells remained stable or even increased in number, whereas in patients who stopped receiving the maintenance vaccinations, the T cells remained present for several months, with a decline thereafter. The ability of these cells to kill melanoma cells was demonstrated ex vivo by transfecting healthy donor CD8+ cells with the cloned TAA-specific T cell receptor from a vaccinated patient and evaluating their ability to lyse melanoma cell lines.
After each dose, increased plasma levels of IFN-α, IFN-γ, IL-6 and other cytokines were found in patients, typically peaking a few hours after injection and normalizing within 24 h. This was in line with the observed adverse event profile, which was characterized by mild-to-moderate flu-like symptoms, equally transient and self-limiting. The first evaluation of 42 patients with radiographically evaluable disease was considered encouraging. In the group of 25 patients with vaccine monotherapy, three had partial responses and seven had stable responses, while six of 17 patients treated with the vaccine–anti-PD1 combination experienced a partial response. An interesting observation was that two patients who had progressed while on anti-PD1 therapy and had received vaccine monotherapy later responded again to anti-PD1 therapy, which is in line with the observation that the induced T cells were of the PD1+ effector memory phenotype. BNT111 is now in a phase 2 study of melanoma (NCT04526899)122. mRNA-5671 (V941) is a concatemer designed to present KRAS antigens to the immune system and codes for the four most common KRAS substitutions (G12D, G12V, G13D, G12C)38. It is currently in a phase 1 study (NCT03948763) as monotherapy and in combination with pembrolizumab10,38,123,124,125.
Additional examples include BNT112 (which encodes five prostate cancer-specific antigens126) and BNT113 (which encodes HPV16-derived tumor antigens E6 and E7 (viral oncoproteins)), BNT114 (ref. 127) (which encodes a mix of selected breast cancer antigens) and BNT115 (which encodes a mixture of three ovarian cancer TAA-encoding RNA species)128.
CureVac conducted early studies with unmodified mRNA species encoding TAAs, including naked mRNA species for autologous amplified tumor mRNA as an immunotherapeutic regimen129, naked mRNA encoding six renal cancer-associated antigens130 and protamine-stabilized mRNA for six different melanoma-associated antigens (NCT00204516,NCT00204607)131. These studies mainly provided safety and tolerability information about the formulations used. Other studies investigating tumor-associated antigens include CV9103 (mixture of four antigens for prostate cancer)132, CV9104 (mixture of six different antigens for prostate cancer encoded by six different mRNA species133 and CV9201 (mixture of five non-small lung cell cancer antigens)134. All these projects and/or drug candidates appear to have ceased development64. CV9202 contains six mRNA species that encode six different antigens (NY-ESO-1, MAGE C1, MAGE C2, trophoblast glycoprotein (TPBG (5T4)), survivin and mucin-1 (MUC1))135 and is still in an active study (NCT03164772)136,137.
Personalized neoantigens
During carcinogenesis, malignant cells acquire somatic mutations that lead to the production of protein sequences not expressed by normal cells138. These proteins are processed via the proteasome into peptides that are presented on the cell surface bound to MHC class I receptors, where they are recognized by T cell receptors. These neoantigens are typically unique to each patient and thus represent both the opportunity for and technical challenges associated with tumor-specific and patient-tailored immunotherapy119.
To generate mRNA vaccines against patient-specific neoantigens, an individual patient’s tumor is excised and patient-specific neoantigens are identified by next-generation sequencing. The mRNA encoding these neoantigens is then injected into the same patient, with the expectation that it will induce an immune response that will attack the patient’s tumor139. It is of course imperative that this entire process should take a minimal amount of time so that the patient can be treated before the cancer evolves and progresses, and turnaround times as short as 30–40 d have been reported128. This poses additional challenges for manufacturing, which must satisfy the criteria for human use of investigational products.
Thus far, the majority of work in personalized neoantigen vaccines has involved the deployment of peptide-based neoantigen vaccines rather than mRNA vaccines; in general, these approaches have had limited success. Tumors with the highest mutational burden, which are in theory the best candidates for this type of neoantigen approach, are also most likely to develop resistance to the treatments140. Compared with peptide vaccines, we hypothesize that mRNA-encoded neoantigen vaccines, with proper immune stimulation, may provide a stronger immunogenic response and clinical benefit. Unlike peptide-based vaccines, mRNA can encode whole antigen, thereby ensuring presentation of multiple epitopes without being restricted to a defined HLA type141. In addition, mRNA can be synthesized to express multiple neoantigens either as separate molecules or as a concatenation of multiple coding sequences. Certain tumor types can produce up to several dozens of neoantigens, and, from the perspective of inducing a broad immunological response, it is desirable to express multiple epitopes likely to evoke a T cell response.
BioNTech has developed several clinical neoantigen vaccine candidates for the treatment of cancer. BNT121 was studied via repeat administration in inguinal lymph nodes of 13 patients with metastatic melanoma (NCT02035956)126,142. The results from that study were considered encouraging, with robust immunological responses and some evidence of clinical activity. BNT122 (RO7198457), which can contain up to 20 individualized patient neoepitopes, is administered i.v. and is currently being evaluated in four studies (Table 2). Preliminary results indicated that BNT122, both with and without the anti-PD-L1 antibody atezolizumab, has an acceptable safety profile with mainly transient adverse events such as infusion-related reaction and/or cytokine-release syndrome manifesting as fever and chills128. BNT122 is also under evaluation in a phase 1 study of pancreatic cancer (NCT04161755), and a study in non-small lung cell cancer is expected to start soon (NCT04267237) as well as a study for an undisclosed adjuvant indication126.
mRNA-4157 is another personalized cancer vaccine that can contain up to 34 neoantigens encoded on a single mRNA strand (‘neoantigen concatemer’) and is formulated in an LNP and administered i.m. This drug is currently in a phase 1 study of patients with resected primary solid tumors (monotherapy) and patients with metastatic unresected tumors (NCT03313778). As of February 2020, a total of 71 patients were reported to have received at least one dose of mRNA-4157 (ref. 38). The most frequently noted adverse events were fatigue, injection-site soreness, colitis and myalgia. In parallel, a randomized phase 2 study as adjuvant in combination with pembrolizumab for patients with high-risk melanoma is also ongoing (NCT03897881). The compound NC-I4650 is closely related to mRNA-4157, the main difference being the neoantigen-selection protocols used38.
Neoantigen vaccines, with their dependence on fast turnaround of patient-specific mRNA sequences, definitely benefit from the flexibility and speed inherent in the mRNA–LNP platform. Lastly, the variety of routes of administration in oncology is worth noting: intratumoral, i.n. and i.v. or i.m., with some of the same LNPs being used for more than one route of administration. This indicates the potential for wide applications of a single drug candidate: a tumor that cannot be reached by direct intratumoral injection or where there are no accessible lymph nodes may still respond to i.v. or i.m. administration of the relevant mRNA vaccine. The challenges are to identify the most effective protein or combination of proteins to encode to direct the immune system to attack cancers, to enable the immune system to penetrate deep into tumors and to personalize the therapies for each patient.
Protein and cellular immunotherapies
An area of renewed interest is the use of mRNA administration with the intent of generating therapeutic levels of immune or immunomodulatory proteins (Table 3), such as antibodies or cytokines. Compared with infectious disease and cancer vaccines, more protein has to be produced for such therapies to be effective, where, in certain cases, life-long treatment with repeated dosing may be required.
Another challenge for protein immunotherapies is delivery of mRNA to the desired organs and cell types to achieve optimal therapeutic outcomes. For instance, certain expressed proteins require further PTMs, such as glycosylation and proteolytic processing, to become fully functional. However, the manner in which PTMs are made to the protein can be tissue dependent and may not be dictated simply by the mRNA sequence, thus emphasizing the need for tissue-specific delivery of the mRNA.
When mRNA species are administered systemically in complex with LNPs, many tend to home to the liver due to binding of apolipoprotein E to the LNP surface, which leads to receptor-mediated uptake by hepatocytes143. Non-liver organ selectivity can be achieved through modification of lipid compositions, including adjusting lipid ratios and identities, leading to LNPs that target the lung endothelium or the spleen144,145. More recently, changes to LNP surface chemistry through modulation of the PEG–lipid structure have led to LNP targeting of bone marrow endothelial cells in the hematopoietic stem cell niche146.
Thus, mRNA protein immunotherapy poses several unique challenges in terms of delivery, efficacy of protein production and tolerability compared with vaccines. This may explain why this application of mRNA has progressed more slowly than mRNA immunization.
mRNA-encoded monoclonal antibody therapy
Delivering an mRNA to a specific tissue or organ by direct injection is a barrier to development. Instead, systemic exposure can simplify clinical application as long as it is safe and a sufficient level of protein is expressed to gain a therapeutic effect. Encoding monoclonal antibodies (mAbs) in an mRNA medicine is an example of this approach and is exemplified by mRNA-1944, an mRNA–LNP encoding a neutralizing mAb against Chikunguya virus, identified in a patient with immunity (NCT03829384)124. Results from the first healthy volunteers treated indicated that, at all doses tested (0.1, 0.3 and 0.6 mg per kg, i.v.), neutralizing mAb levels could be detected. At the highest dose, however, three of four participants experienced infusion-related reactions, including grade 3 tachycardia and elevated white blood cell count in one participant, who also had grade 2 nausea, emesis, fever and transient inverted T waves on electrocardiogram147. A separate cohort at that same dose level but pretreated with steroids had no grade 3 adverse events, but the levels of Chikungunya-specific antibodies produced (Emax) were 1.7-fold lower67. Data from a cohort to which a dose of 0.3 mg per kg was administered twice, 2 weeks apart, indicated no exacerbation of adverse events after the first versus second dose and no lipid accumulation67.
The application of mRNA to produce antibodies continues to be of interest, with several industry collaborations underway, such as partnerships between CureVac and Genmab (mRNA-based antibody anti-cancer therapeutics148) and between Neurimmune and Ethris (inhaled mRNA encoding mAbs against SARS-CoV-2 (ref. 149)). An important consideration here are the benefits of expressing an mAb from an mRNA, rather than administering the same antibody made through traditional recombinant manufacturing. Ultimately, the most promising approach will be a function of the doses required, the duration of effect, the types of PTMs needed and the relative therapeutic index ratio of the delivery system and the antibody of interest.
mRNA-encoded immunostimulatory proteins for cancer treatment
Another anti-cancer approach consists of the injection of mRNA encoding proteins expected to have a direct therapeutic effect, typically via stimulating the immune system, such as OX40 ligand (OX40L) or ILs. One such product, mRNA-2416, is an mRNA encoding the immune checkpoint modulator OX40L, administered intratumorally. Despite the first reported results as monotherapy in 41 patients with a variety of malignancies not meeting the Response Evaluation Criteria in Solid Tumors for a partial response, the sponsor is currently taking it forward into a phase 2 expansion cohort in combination with durvalumab for ovarian cancer (NCT03323398)38.
Other mRNA products encode several different immunomodulatory proteins. One example is ECI-006, a combination of TriMix (mRNA species encoding DC-activating molecules (CD40L, CD70 and caTLR4)) and mRNA species encoding melanoma-specific TAAs (tyrosinase, gp100, MAGE A3, MAGE C2 and PRAME)150, which is administered i.n. and is being tested in a phase 1 study of resected melanoma (NCT03394937)123 (TriMix alone is in a phase 1 study of breast cancer (NCT03788083) and given intratumorally151). An additional example is mRNA-2752 (three mRNA species encoding OX40L, IL-23 and IL-36γ), which is being evaluated in a dose-escalation study of solid tumors and lymphoma (NCT03739931). Similarly, BNT131 (SAR441000) encodes IL-12sc, IL-15sushi, IFN-α and granulocyte–macrophage colony-stimulating factor (GM-CSF) and is under investigation as an intratumoral injection intended to alter the tumor microenvironment128.
Another type of product is immunomodulatory fusion proteins. MEDI1191 encodes a single chain fusion protein containing the IL-12α and IL-12β subunits, with a linker between the subunits. This agent was developed for intratumoral injection, with the aim of improved tolerability compared with systemic administration of recombinant IL-12 (ref. 38).
mRNA in adoptive immune cell therapy
Adoptive cell transfer is a relatively new therapeutic approach that involves collecting and using a patient’s own immune cells to treat their cancer152. This has been explored in humans with breast cancer for which Tchou et al.153 observed that intratumoral injections of T cells transfected with mRNA encoding a chimeric antigen receptor (CAR) targeting c-Met were well tolerated and induced an inflammatory response within breast cancer tumor tissue; similarly, Maus et al.154 reported on four individuals treated with autologous T cells electroporated with mRNA encoding a CAR derived from a murine antibody specific to human mesothelioma. One of the treated individuals experienced anaphylaxis and cardiac arrest within minutes after the third infusion, which the authors attributed to the production of immunoglobulin (Ig)E-type human anti-mouse antibodies (NCT01355965). More recently, Beatty et al.155 evaluated T cells transfected with an mRNA encoding a mesothelin-directed CAR as a treatment for pancreatic cancer that avoided T cell priming; in phase 1 studies, these cells did not induce cytokine-release syndrome and did not elicit neurologic symptoms.
CAR T cells have been historically generated using retroviral gene transfer with substantial success and, more recently, using CRISPR–Cas9-mediated gene-integration systems. Success has been reported using not only mRNA but also ribonucleoprotein-mediated delivery systems. However, as discussed above, we see the potential of CAR T cell generation in vivo156. Although functional delivery of ribonucleoproteins in vivo has yet to be described, ultimately, mRNA may have substantial advantages over viral delivery in terms of both loading capacity and redosing, assuming safe and effective delivery to T cells can be demonstrated.
Nanoformulations capable of facilitating in vivo delivery to multiple classes of immune cells have also been described, including macrophages157, B cells158 and T cells156,159,160,161, offering the promise of a range of immunotherapies. For example, with T cell-targeted mRNA delivery, in vivo CAR T cell generation may be possible, creating new types of therapy for cancer156. Retargeting LNPs to T cells has been achieved through the identification of specific lipid structures that facilitate delivery to these cells160,161. Furthermore, antibody-targeted mRNA nanoparticles, for which specificity is imparted by surface conjugation, enable affinity to immune cell-specific receptors, such as CD4 (ref. 162).
For therapies for which the aim is to edit immune cells in vivo, mRNA may allow transient expression of the genome-editing nucleases or base editors, which last transiently in cells due to RNA degradation. Although, thus far, published reports of mRNA delivery to T cells have only shown disruption of green fluorescent protein marker in vitro159, the success of gene editing via systemically delivered mRNA to hepatocytes has already been demonstrated in humans for TTR amyloidosis, for which an mRNA encoding Cas9 protein has been delivered in an LNP together with a guide RNA targeting TTR. In patients, there was a dose-dependent mean reduction from baseline in serum TTR protein concentrations with only mild adverse events163. This impressive outcome hints at the future potential use of mRNA in systemic gene editing.
Conclusions and future directions
mRNA occupies a distinctive niche between gene therapy and protein therapy, combining many of the advantages of both while addressing unique challenges faced by either one. For instance, multimeric proteins that would pose insurmountable technical challenges for production in a bioreactor can be produced in the patient’s own body by an mRNA or combination of mRNA species encoding the different subunits, an opportunity for flexibility that has been put to use in a candidate CMV vaccine and for cancer applications and that takes on a new importance as the world is grappling with the emergence of distinct SARS-CoV-2 strains with anticipated different sensitivities to the authorized SARS-CoV-2 vaccines. As the field optimizes and refines the technology, it is likely that mRNA medicines will also be developed for indications beyond infectious disease and cancer.
The inherently transient duration of the expression of the target protein positions mRNA therapy as an ideal modality for situations in which a single or no more than a small number of episodes of protein expression are required, such as infectious disease vaccines. The ability to dose repeatedly, titrate the dose or vary the dosing interval offers the clinician the flexibility of classic drug therapy, making it an attractive choice for indications for which individual patient needs may vary or for which hesitations may exist about gene therapy. From the safety point of view, for two vaccine projects (mRNA-1273 for SARS-CoV-2 and mRNA-1647), it appeared that there was a more pronounced adverse event profile after the second dose than after the first dose. This does not seem to be a universal observation, however. The cancer vaccine BNT111 has been administered to some patients with more than eight doses, with apparently maintained efficacy15. The inhaled mRNA therapeutic MRT5005 has been given in up to five weekly doses, with no signs of worsening safety profile between the first and the fifth dose.
The potential of mRNA therapies will expand further through the evaluation of engineered, non-human and artificial protein constructs. Protein therapeutics have been engineered for extended half-life, for example, through fusion of the Fc domain to the therapeutic domain. The same can be encoded in mRNA therapies. Perhaps more exciting is the ability to express new intracellular therapeutics. The transient expression of gene-editing machinery from mRNA is appealing to reduce side effects from persistent expression. Additionally, the intracellular expression of antibodies, antibody fragments or other protein-binding motifs provides a distinct therapeutic class that can be combined with subcellular localization domains, for example, to the nucleus, to focus the action of the encoded protein.
The first applications of mRNA involved stimulation of the immune system, either for infectious disease vaccines or for cancer vaccines. The infectious disease application has been a proving ground for the platform; cancer vaccines have hitherto not been particularly successful as a class, but the encouraging results of BNT111 hint at the possibility that the combination of high protein expression and the immune-activation pathway may overcome some of the hurdles encountered by earlier protein vaccines.
Exploiting the immunostimulatory properties of RNA in products makes perfect sense thanks to the intrinsic ability of RNA to activate immune pathways via the TLR and RIG-I pathways. This is independent of the coded protein, as shown by CV8102, a noncoding RNA that is used as an immune adjuvant. The drawback of this immunostimulatory property is manifest upon a review of the safety and tolerability profiles of the mRNA drug candidates that have entered clinical trials. The emerging picture is that the most frequently noted adverse event associated with mRNA medicines as a class is some form of inflammatory reaction. This underlying pathway can manifest in a large number of ways: a local reaction in the case of i.m. or subcutaneous injection (local pain, redness, soreness) or as a more generalized febrile syndrome or flu-like reaction with i.v., i.m. or inhaled medicines. These seem to be typically treatable with classic anti-inflammatory drugs, although, in the case of i.v. administration of mRNA encoding a Chikungunya-specific mAb, prophylactic steroid use was used to mitigate the adverse events observed in the cohort receiving the highest dose67. This intervention did appear to be successful in tamping down the adverse events but was also associated with a decrease in protein expression.
These data offer interesting insights into some potential future directions for research into repeat dosing of mRNA therapeutics. Will steroids be effective and required for the mitigation of inflammatory side effect profiles of mRNA-encoded protein therapies? And if so, does that come at a price of less protein expression? Does the concomitant observation of fewer side effects with reduced expression hint that a certain level of inflammation might actually be a prerequisite for good protein expression? If this is the case, one clinical challenge will be to thread that needle and allow just enough subclinical inflammatory processes to be initiated to promote good translation without allowing them to rise to levels of severity that would jeopardize the clinical feasibility of repeat dosing.
These questions are somewhat complicated by the fact that the majority of mRNA applications do not involve simple naked mRNA but mRNA that is encapsulated in an LNP or PNP, all of which can contribute to the tolerability profile. The use of ‘empty LNP’ containing no mRNA in a control arm of clinical trials has been proposed to help elucidate the distinct contributions of mRNA versus LNP to the tolerability profile. A theoretical limitation to this approach is that the empty LNPs, when not complexed with negatively charged mRNA, have different physicochemical properties and thus do not represent a true comparator. Although a transient inflammatory reaction can be acceptable in the context of a single dose (vaccination) or life-threatening diseases (oncology), for indications for which chronic treatment is necessary, especially when administered i.v., the selection of appropriate, well-tolerated and safe lipid and formulation will be critical. It appears that animal experimentation will be of limited value here because it was noted that the concentrations of the cancer vaccine BNT111 that triggered cytokine release in humans were >1,000-fold lower in humans than those in mice15, and the febrile reactions observed after inhalation of MRT5005 were not predicted based on animal toxicity experiments.
Beyond the immediate tolerability, longer-term questions about the potential for lipid accumulation will also have to be addressed. If the produced protein has a short half-life, then the dosing interval required to maintain expression and clinical efficacy may be shorter than ideal for elimination of the lipid. Repeat dosing could thus lead to lipid accumulation in target or off-target tissues, with difficult-to-predict long-term safety risks. Formulation science will be as much a part of the future of mRNA therapeutics as further understanding of the biology of mRNA itself.
The extraordinary potential of mRNA therapy is also illustrated by the different routes of administration introduced into the clinic: i.m., i.d., subcutaneous, i.n., intratumoral, i.v., epicardial and inhaled. Additional applications can readily be imagined: intranasal vaccines, eye or nose drops, skin ointments, suppositories, solutions for intravesical instillation, intrathecal drug-delivery devices or Ommaya reservoirs. We believe that the future breadth of mRNA therapy will be defined by advances in delivery nanoparticles. There is growing evidence that LNPs and PNPs can be engineered to deliver to a range of tissues in the body including the liver65,72, the endothelium144, the lung81,82,83, the bone marrow146 and multiple elements of the immune system156,157,158,159,160,161,162. Additional advances in potency and tissue targeting by improved delivery materials and inclusion of additional targeting elements will continue to open doors to new therapeutic applications for mRNA therapy.
The success of the two SARS-CoV-2 vaccines receiving EUAs has highlighted one of the pharmaceutical advantages of mRNA: the speed of production of clinical trial materials. Manufacturing technologies originally developed for the fast turnaround of individualized neoantigen vaccines from excised patient tumors toward an injection-ready clinical product have demonstrated their potential by enabling the start of clinical trials of candidate mRNA vaccines within weeks after the publication of the SARS-CoV-2 sequence.
At the same time, our experience with COVID-19 vaccines also highlights one of the current limitations of the technology: the dependency on cold-chain storage and transport. Freezers capable of handling temperatures of −80 °C are specialized equipment and not readily available in every pharmacy or clinical trial site. For treatments that are intended for self-administration in the patient’s home, even storage at −20 °C can be challenging. The next frontier to bring mRNA medicines from the bench to the bedside may very well be formulation science.
The emergence of mRNA as a safe and effective platform in the race to produce a COVID-19 vaccine has provided the entire world with an accelerated education in the benefits and risks of mRNA–LNP technology. Although much attention has been lavished on the speed of manufacturing and storage conditions of BNT121 and mRNA-1273 as well as the side effects of a two-dose regimen, the scientific and medical communities are looking beyond these two vaccines and eagerly awaiting further validation of mRNA medicines in other indications164,165.
References
- Moderna. Moderna’s Work on a Potential Vaccine Against COVID-19 https://www.modernatx.com/modernas-work-potential-vaccine-against-covid-19 (2020).
- Corbett, K. S. et al. SARS-CoV-2 mRNA vaccine design enabled by prototype pathogen preparedness. Nature 586, 567–571 (2020).CAS PubMed PubMed Central Article Google Scholar
- Wolff, J. A. et al. Direct gene transfer into mouse muscle in vivo. Science 247, 1465–1468 (1990).CAS PubMed Article Google Scholar
- Kutzler, M. A. & Weiner, D. B. DNA vaccines: ready for prime time? Nat. Rev. Genet. 9, 776–788 (2008).CAS PubMed PubMed Central Article Google Scholar
- Cross, R. Can mRNA disrupt the drug industry? Chem. Eng. News 96, 1–14 (2018).Google Scholar
- Van Hoecke, L. & Roose, K. How mRNA therapeutics are entering the monoclonal antibody field. J. Transl. Med. 17, 54 (2019).PubMed PubMed Central Article Google Scholar
- Maruggi, G., Zhang, C., Li, J., Ulmer, J. B. & Yu, D. mRNA as a transformative technology for vaccine development to control infectious diseases. Mol. Ther. 27, 757–772 (2019).CAS PubMed PubMed Central Article Google Scholar
- Schlake, T., Thess, A., Thran, M. & Jordan, I. mRNA as novel technology for passive immunotherapy. Cell. Mol. Life Sci. 76, 301–328 (2019).CAS PubMed Article Google Scholar
- Jackson, N. A. C., Kester, K. E., Casimiro, D., Gurunathan, S. & DeRosa, F. The promise of mRNA vaccines: a biotech and industrial perspective. NPJ Vaccines 5, 11 (2020).PubMed PubMed Central Article Google Scholar
- Kowalski, P. S., Rudra, A., Miao, L. & Anderson, D. G. Delivering the messenger: advances in technologies for therapeutic mRNA delivery. Mol. Ther. 27, 710–728 (2019).CAS PubMed PubMed Central Article Google Scholar
- Colella, P., Ronzitti, G. & Mingozzi, F. Emerging issues in AAV-mediated in vivo gene therapy. Mol. Ther. Methods Clin. Dev. 8, 87–104 (2018).CAS PubMed Article Google Scholar
- Sabnis, S. et al. A novel amino lipid series for mRNA delivery: improved endosomal escape and sustained pharmacology and safety in non-human primates. Mol. Ther. 26, 1509–1519 (2018).CAS PubMed PubMed Central Article Google Scholar
- Chahal, J. S. et al. Dendrimer-RNA nanoparticles generate protective immunity against lethal Ebola, H1N1 influenza, and Toxoplasma gondii challenges with a single dose. Proc. Natl Acad. Sci. USA 113, E4133–4142 (2016).CAS PubMed PubMed Central Article Google Scholar
- Besin, G. et al. Accelerated blood clearance of lipid nanoparticles entails a biphasic humoral response of B-1 followed by B-2 lymphocytes to distinct antigenic moieties. Immunohorizons 3, 282–293 (2019).CAS PubMed Article Google Scholar
- Sahin, U. et al. An RNA vaccine drives immunity in checkpoint-inhibitor-treated melanoma. Nature 585, 107–112 (2020).CAS PubMed Article Google Scholar
- Adams, D. et al. Patisiran, an RNAi therapeutic, for hereditary transthyretin amyloidosis. N. Engl. J. Med. 379, 11–21 (2018).CAS PubMed Article Google Scholar
- Kariko, K. et al. Incorporation of pseudouridine into mRNA yields superior nonimmunogenic vector with increased translational capacity and biological stability. Mol. Ther. 16, 1833–1840 (2008).CAS PubMed Article Google Scholar
- Thess, A. et al. Sequence-engineered mRNA without chemical nucleoside modifications enables an effective protein therapy in large animals. Mol. Ther. 23, 1456–1464 (2015).CAS PubMed PubMed Central Article Google Scholar
- Guan, S. & Rosenecker, J. Nanotechnologies in delivery of mRNA therapeutics using nonviral vector-based delivery systems. Gene Ther. 24, 133–143 (2017).CAS PubMed Article Google Scholar
- Kauffman, K. J., Webber, M. J. & Anderson, D. G. Materials for non-viral intracellular delivery of messenger RNA therapeutics. J. Control. Release 240, 227–234 (2016).CAS PubMed Article Google Scholar
- Dorrie, J., Schaft, N., Schuler, G. & Schuler-Thurner, B. Therapeutic cancer vaccination with ex vivo RNA-transfected dendritic cells—an update. Pharmaceutics 12, 92 (2020).
- Pardi, N., Hogan, M. J., Porter, F. W. & Weissman, D. mRNA vaccines—a new era in vaccinology. Nat. Rev. Drug Discov. 17, 261–279 (2018).CAS PubMed PubMed Central Article Google Scholar
- Hajj, K. A. & Whitehead, K. A. Tools for translation: non-viral materials for therapeutic mRNA delivery. Nat. Rev. Mater. 2, 17056 (2017).CAS Article Google Scholar
- Sahin, U., Kariko, K. & Tureci, O. mRNA-based therapeutics—developing a new class of drugs. Nat. Rev. Drug Discov. 13, 759–780 (2014).CAS PubMed Article Google Scholar
- Weng, Y. et al. The challenge and prospect of mRNA therapeutics landscape. Biotechnol. Adv. 40, 107534 (2020).
- Zhang, C., Maruggi, G., Shan, H. & Li, J. Advances in mRNA vaccines for infectious diseases. Front. Immunol. 10, 594 (2019).CAS PubMed PubMed Central Article Google Scholar
- Tatematsu, M., Funami, K., Seya, T. & Matsumoto, M. Extracellular RNA sensing by pattern recognition receptors. J. Innate Immun. 10, 398–406 (2018).CAS PubMed PubMed Central Article Google Scholar
- Frega, G. et al. Trial Watch: experimental TLR7/TLR8 agonists for oncological indications. Oncoimmunology 9, 1796002 (2020).PubMed PubMed Central Article Google Scholar
- CureVac. CureVac Provides Update on Phase 2b/3 Trial of First-Generation COVID-19 Vaccine Candidate, CVnCoV https://www.curevac.com/en/2021/06/16/curevac-provides-update-on-phase-2b-3-trial-of-first-generation-covid-19-vaccine-candidate-cvncov/ (2021).
- Cohen, J. What went wrong with CureVac’s mRNA vaccine? Science 372, 1381 (2021).CAS Article Google Scholar
- Chandrashekar, A. et al. SARS-CoV-2 infection protects against rechallenge in rhesus macaques. Science 369, 812–817 (2020).CAS PubMed PubMed Central Article Google Scholar
- Yu, J. et al. DNA vaccine protection against SARS-CoV-2 in rhesus macaques. Science 369, 806–811 (2020).
- BioNTech. Pfizer and BioNTech Celebrate Historic First Authorization in the U.S. of Vaccine to Prevent COVID-19 https://investors.biontech.de/node/8926/pdf (2020).
- Moderna. U.S. CDC Advisory Committee on Immunization Practices Recommends Vaccination with Moderna’s COVID-19 Vaccine for Persons 18 Years and Older https://investors.modernatx.com/news/news-details/2020/U.S.-CDC-Advisory-Committee-on-Immunization-Practices-Recommends-Vaccination-with-Modernas-COVID-19-Vaccine-for-Persons-18-Years-and-Older-12-19-2020/default.aspx (2020).
- CureVac. CureVac Receives Regulatory Approval from German and Belgian Authorities to Initiate Phase 1 Clinical Trial of its SARS-CoV-2 Vaccine Candidate https://www.curevac.com/wp-content/uploads/2020/08/20200617_PR_CureVac_Approval_Phase1_EN_final.pdf?x37733 (2020).
- Arcturus. Arcturus Therapeutics Announces Positive Interim ARCT-021 (LUNAR-COV19) Phase 1/2 Study Results for both Single Shot and Prime–Boost Regimens, and up to $220 Million in Additional Financial Commitments from Singapore https://ir.arcturusrx.com/node/10176/pdf (2020).
- Translate Bio. Sanofi Pasteur and Translate Bio to Collaborate to Develop a Novel mRNA Vaccine Candidate Against COVID-19 https://investors.translate.bio/node/7396/pdf (2020).
- Moderna, Inc. United States Securities and Exchange Commission Form 10-K (2020).
- Brisse, M., Vrba, S. M., Kirk, N., Liang, Y. & Ly, H. Emerging concepts and technologies in vaccine development. Front. Immunol. 11, 583077 (2020).CAS PubMed PubMed Central Article Google Scholar
- Moderna. Moderna Announces Progress Across Broad Portfolio and all Three Clinical Stage Therapeutic Areas at 2020 R&D Day https://investors.modernatx.com/node/9896/pdf (2020).
- Aldrich, C. et al. Proof-of-concept of a low-dose unmodified mRNA-based rabies vaccine formulated with lipid nanoparticles in human volunteers: a phase 1 trial. Vaccine 39, 1310–1318 (2021).CAS PubMed PubMed Central Article Google Scholar
- Buschmann, M. D. et al. Nanomaterial delivery systems for mRNA vaccines. Vaccines 9, 65 (2021).
- Kis, Z., Shattock, R., Shah, N. & Kontoravdi, C. Emerging technologies for low-cost, rapid vaccine manufacture. Biotechnol. J. 14, e1800376 (2019).PubMed Article CAS Google Scholar
- Rauch, S., Jasny, E., Schmidt, K. E. & Petsch, B. New vaccine technologies to combat outbreak situations. Front. Immunol. 9, 1963 (2018).PubMed PubMed Central Article CAS Google Scholar
- Mulligan, M. J. et al. Phase I/II study of COVID-19 RNA vaccine BNT162b1 in adults. Nature 586, 589–593 (2020).CAS PubMed Article Google Scholar
- Walsh, E. E. et al. Safety and immunogenicity of two RNA-based COVID-19 vaccine candidates. N. Engl. J. Med. 383, 2439–2450 (2020).CAS PubMed Article Google Scholar
- Kremsner, P. et al. Safety and immunogenicity of an mRNA-lipid nanoparticle vaccine candidate against SARS-CoV-2: a phase 1 randomized clinical trial. Wien Klin. Wochenschr. 133, 931–941 (2021).CAS PubMed PubMed Central Article Google Scholar
- Jackson, L. A. et al. An mRNA vaccine against SARS-CoV-2—preliminary report. N. Engl. J. Med. 383, 1920–1931 (2020).CAS PubMed Article Google Scholar
- Kalnin, K.V., Plitnik, T. & Kishko, M. et al. Immunogenicity and efficacy of mRNA COVID-19 vaccine MRT5500 in preclinical animal models. NPJ Vaccines 6, 61 (2021).CAS PubMed PubMed Central Article Google Scholar
- Aliprantis, A. O. et al. A phase 1, randomized, placebo-controlled study to evaluate the safety and immunogenicity of an mRNA-based RSV prefusion F protein vaccine in healthy younger and older adults. Hum. Vaccin. Immunother. 17, 1248–1261 (2020).
- Moderna. First Vaccines Day https://investors.modernatx.com/events-and-presentations/events/event-details/2020/Vaccine-Day/default.aspx (2020).
- Kalathiya, U. et al. Highly conserved homotrimer cavity formed by the SARS-CoV-2 spike glycoprotein: a novel binding site. J. Clin. Med. 9, 1473 (2020).CAS PubMed Central Article Google Scholar
- Edwards, D. K. et al. Adjuvant effects of a sequence-engineered mRNA vaccine: translational profiling demonstrates similar human and murine innate response. J. Transl. Med. 15, 1 (2017).PubMed PubMed Central Article CAS Google Scholar
- Weissman, D. et al. HIV Gag mRNA transfection of dendritic cells (DC) delivers encoded antigen to MHC class I and II molecules, causes DC maturation, and induces a potent human in vitro primary immune response. J. Immunol. 165, 4710–4717 (2000).CAS PubMed Article Google Scholar
- Lutz, J. et al. Unmodified mRNA in LNPs constitutes a competitive technology for prophylactic vaccines. NPJ Vaccines 2, 29 (2017).
- Anderson, B. R. et al. Incorporation of pseudouridine into mRNA enhances translation by diminishing PKR activation. Nucleic Acids Res. 38, 5884–5892 (2010).CAS PubMed PubMed Central Article Google Scholar
- Karikó, K., Buckstein, M., Ni, H. & Weissman, D. Suppression of RNA recognition by Toll-like receptors: the impact of nucleoside modification and the evolutionary origin of RNA. Immunity 23, 165–175 (2005).PubMed Article CAS Google Scholar
- World Health Organization. Messenger RNA Encoding the Full-Length SARS-CoV-2 Spike Glycoprotein https://berthub.eu/articles/11889.doc (2020).
- Kauffman, K. J. et al. Efficacy and immunogenicity of unmodified and pseudouridine-modified mRNA delivered systemically with lipid nanoparticles in vivo. Biomaterials 109, 78–87 (2016).CAS PubMed PubMed Central Article Google Scholar
- Kariko, K., Muramatsu, H., Ludwig, J. & Weissman, D. Generating the optimal mRNA for therapy: HPLC purification eliminates immune activation and improves translation of nucleoside-modified, protein-encoding mRNA. Nucleic Acids Res. 39, e142 (2011).CAS PubMed PubMed Central Article Google Scholar
- Baiersdörfer, M. et al. A facile method for the removal of dsRNA contaminant from in vitro-transcribed mRNA. Mol. Ther. Nucleic Acids 15, 26–35 (2019).PubMed PubMed Central Article CAS Google Scholar
- Tahtinen, S. et al. IL-1 and IL-1ra are key regulators of the inflammatory response to RNA vaccines. Nat. Immunol. 23, 532–542 (2022).CAS PubMed Article Google Scholar
- Alberer, M. et al. Safety and immunogenicity of a mRNA rabies vaccine in healthy adults: an open-label, non-randomised, prospective, first-in-human phase 1 clinical trial. Lancet 390, 1511–1520 (2017).CAS PubMed Article Google Scholar
- CureVac. CureVac B.V. Securities and Exchange Commission Filing https://sec.report/Document/0001104659-20-086354/tm2016252-11_f1.htm (2020).
- Zhang, X., Goel, V. & Robbie, G. J. Pharmacokinetics of patisiran, the first approved RNA interference therapy in patients with hereditary transthyretin-mediated amyloidosis. J. Clin. Pharmacol. 60, 573–585 (2019).PubMed Central Google Scholar
- BioNTech. Update on COVID-19 Vaccine Program https://investors.biontech.de/events/event-details/update-covid-19-vaccine-program-press-conference-0 (2020).
- Moderna. Annual R&D Day https://investors.modernatx.com/events-and-presentations/events/event-details/2020/RD-Day/default.aspx (2020).
- Akinc, A. et al. A combinatorial library of lipid-like materials for delivery of RNAi therapeutics. Nat. Biotechnol. 26, 561–569 (2008).CAS PubMed PubMed Central Article Google Scholar
- Love, K. T. et al. Lipid-like materials for low-dose, in vivo gene silencing. Proc. Natl Acad. Sci. USA 107, 1864–1869 (2010).
- Dong, Y. et al. Lipopeptide nanoparticles for potent and selective siRNA delivery in rodents and nonhuman primates. Proc. Natl Acad. Sci. USA 111, 3955–3960 (2014).CAS PubMed PubMed Central Article Google Scholar
- Whitehead, K. A. et al. Degradable lipid nanoparticles with predictable in vivo siRNA delivery activity. Nat. Commun. 5, 4277 (2014).CAS PubMed Article Google Scholar
- Fenton, O. S. et al. Bioinspired alkenyl amino alcohol ionizable lipid materials for highly potent in vivo mRNA delivery. Adv. Mater. 28, 2939–2943 (2016).CAS PubMed PubMed Central Article Google Scholar
- Maier, M. A. et al. Biodegradable lipids enabling rapidly eliminated lipid nanoparticles for systemic delivery of RNAi therapeutics. Mol. Ther. 21, 1570–1578 (2013).CAS PubMed PubMed Central Article Google Scholar
- Hassett, K. J. et al. Optimization of lipid nanoparticles for intramuscular administration of mRNA vaccines. Mol. Ther. Nucleic Acids 15, 1–11 (2019).CAS PubMed PubMed Central Article Google Scholar
- Nabhan, J. F. et al. Intrathecal delivery of frataxin mRNA encapsulated in lipid nanoparticles to dorsal root ganglia as a potential therapeutic for Friedreich’s ataxia. Sci. Rep. 6, 20019 (2016).
- Patel, S., Ryals, R. C., Weller, K. K., Pennesi, M. E. & Sahay, G. Lipid nanoparticles for delivery of messenger RNA to the back of the eye. J. Control. Release 303, 91–100 (2019).CAS PubMed PubMed Central Article Google Scholar
- Lewin, A. S. et al. The effects of PEGylation on LNP based mRNA delivery to the eye. PLoS ONE 15, e0241006 (2020).
- Turnbull, I. C. et al. Myocardial delivery of lipidoid nanoparticle carrying modRNA induces rapid and transient expression. Mol. Ther. 24, 66–75 (2016).CAS PubMed Article Google Scholar
- Anttila, V. et al. Synthetic mRNA encoding VEGF-A in patients undergoing coronary artery bypass grafting: design of a phase 2a clinical trial. Mol. Ther. Methods Clin. Dev. 18, 464–472 (2020).CAS PubMed PubMed Central Article Google Scholar
- Sahu, I., Haque, A. K. M. A., Weidensee, B., Weinmann, P. & Kormann, M. S. D. Recent developments in mRNA-based protein supplementation therapy to target lung diseases. Mol. Ther. 27, 803–823 (2019).
- Patel, A. K. et al. Inhaled nanoformulated mRNA polyplexes for protein production in lung epithelium. Adv. Mater. 31, e1805116 (2019).PubMed PubMed Central Article CAS Google Scholar
- Lokugamage, M. P. et al. Optimization of lipid nanoparticles for the delivery of nebulized therapeutic mRNA to the lungs. Nat. Biomed. Eng. 5, 1059–1068 (2021).CAS PubMed Article Google Scholar
- DeRosa, F., Heartlein, M. & Karve, S. Ice-Based Lipid Nanoparticle Formulation for Delivery of mRNA https://patents.google.com/patent/US20200155691A1/ (2020).
- CureVac. CureVac’s COVID-19 Vaccine Candidate, CVnCoV, Suitable for Standard Fridge Temperature Logistics https://www.curevac.com/en/2020/11/12/curevacs-covid-19-vaccine-candidate-cvncov-suitable-for-standard-fridge-temperature-logistics/ (2020).
- Moderna. Moderna Announces Longer Shelf Life for its COVID-19 Vaccine Candidate at Refrigerated Temperatures https://investors.modernatx.com/news/news-details/2020/Moderna-Announces-Longer-Shelf-Life-for-its-COVID-19-Vaccine-Candidate-at-Refrigerated-Temperatures-11-16-2020/default.aspx (2020).
- US FDA. Coronavirus (COVID-19) Update: FDA Allows More Flexible Storage, Transportation Conditions for Pfizer–BioNTech COVID-19 Vaccine https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-allows-more-flexible-storage-transportation-conditions-pfizer#:~:text=Today%2C%20the%20U.S.%20Food%20and,of%20up%20to%20two%20weeks (2021).
- Pfizer. Pfizer and BioNTech Submit COVID-19 Vaccine Stability Data at Standard Freezer Temperature to the U.S. FDA https://www.pfizer.com/news/press-release/press-release-detail/pfizer-and-biontech-submit-covid-19-vaccine-stability-data (2021).
- Moderna. Moderna Provides Mid-Year Corporate Update https://investors.modernatx.com/news/news-details/2018/Moderna-Provides-Mid-Year-Corporate-Update-07-11-2018/default.aspx (2018).
- Moderna, Inc. United States Securities and Exchange Commission Form S-1 (2018).
- Zhao, P. et al. Long-term storage of lipid-like nanoparticles for mRNA delivery. Bioact. Mater. 5, 358–363 (2020).PubMed PubMed Central Article Google Scholar
- Crommelin, D. J. A., Anchordoquy, T. J., Volkin, D. B., Jiskoot, W. & Mastrobattista, E. Addressing the cold reality of mRNA vaccine stability. J. Pharm. Sci. 110, 997–1001 (2020).
- O’Hare, R. Landmark coronavirus study to trial inhaled Imperial and Oxford vaccines. Imperial News Imperial College London https://www.imperial.ac.uk/news/203653/landmark-coronavirus-study-trial-inhaled-imperial/ (14 September 2020).
- Wang, Z. et al. Enhanced SARS-CoV-2 neutralization by dimeric IgA. Sci. Transl. Med. 13, eabf1555 (2021).
- Pizzolla, A. et al. Resident memory CD8+ T cells in the upper respiratory tract prevent pulmonary influenza virus infection. Sci. Immunol. 2, eaam6970 (2017).
- Allie, S. R. et al. The establishment of resident memory B cells in the lung requires local antigen encounter. Nat. Immunol. 20, 97–108 (2018).PubMed PubMed Central Article CAS Google Scholar
- Lorenzi, J. C. C. et al. Intranasal vaccination with messenger RNA as a new approach in gene therapy: use against tuberculosis. BMC Biotechnology 10, 77 (2010).
- Feldman, R. A. et al. mRNA vaccines against H10N8 and H7N9 influenza viruses of pandemic potential are immunogenic and well tolerated in healthy adults in phase 1 randomized clinical trials. Vaccine 37, 3326–3334 (2019).CAS PubMed Article Google Scholar
- Leal, L. et al. Phase I clinical trial of an intranodally administered mRNA-based therapeutic vaccine against HIV-1 infection. AIDS 32, 2533–2545 (2018).CAS PubMed Article Google Scholar
- Thomas, K. U.S. COVID vaccine supply: how to make sense of those confusing numbers. The New York Times https://www.nytimes.com/2021/01/21/health/covid-vaccine-supply-biden.html (21 January 2021).
- Parry, H. et al. Extended interval BNT162b2 vaccination enhances peak antibody generation. NPJ Vaccines 7, 14 (2022).CAS PubMed PubMed Central Article Google Scholar
- Thomas, S. J. et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine through 6 months. N. Engl. J. Med. 385, 1761–1773 (2021).CAS PubMed Article Google Scholar
- Moderna. Moderna Announces Advances Across Its Industry Leading mRNA Pipeline and Provides Business Update https://investors.modernatx.com/news/news-details/2022/Moderna-Announces-Advances-Across-Its-Industry-Leading-mRNA-Pipeline-and-Provides-Business-Update-01-10-2022/default.aspx (2022).
- Baden, L. R. et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N. Engl. J. Med. 384, 403–416 (2021).CAS PubMed Article Google Scholar
- Polack, F. P. et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N. Engl. J. Med. 383, 2603–2615 (2020).CAS PubMed Article Google Scholar
- Pfizer. Pfizer and BioNTech Conclude Phase 3 Study of COVID-19 Vaccine Candidate, Meeting All Primary Efficacy Endpoints https://www.pfizer.com/news/press-release/press-release-detail/pfizer-and-biontech-conclude-phase-3-study-covid-19-vaccine (2020).
- CureVac. CureVac Commences Global Pivotal Phase 2b/3 Trial for COVID-19 Vaccine Candidate, CVnCoV https://www.curevac.com/en/2020/12/14/curevac-commences-global-pivotal-phase-2b-3-trial-for-covid-19-vaccine-candidate-cvncov/ (2020).
- CureVac. CureVac Announces Positive Results in Low Dose—1 μg—Rabies Vaccine Clinical Phase 1 Study https://www.curevac.com/en/curevac-announces-positive-results-in-low-dose-1-%c2%b5g-rabies-vaccine-clinical-phase-1-study/ (2020).
- Doener, F. et al. RNA-based adjuvant CV8102 enhances the immunogenicity of a licensed rabies vaccine in a first-in-human trial. Vaccine 37, 1819–1826 (2019).CAS PubMed Article Google Scholar
- Eigentler, T., Krauss, J. & Schriber, J. Intratumoral RNA-based TLR-7/-8 and RIG-I agonist CV8102 alone and in combination with anti-PD-1 in a phase I dose-escalation and expansion trial in patients with advanced solid tumors. Cancer Res. 79, LB-021 (2019).
- Kallen, K. J. et al. A novel, disruptive vaccination technology: self-adjuvanted RNActive® vaccines. Hum. Vaccin. Immunother. 9, 2263–2276 (2013).CAS PubMed PubMed Central Article Google Scholar
- Rauch, S., Lutz, J., Kowalczyk, A., Schlake, T. & Heidenreich, R. RNActive® technology: generation and testing of stable and immunogenic mRNA vaccines. Methods Mol. Biol. 1499, 89–107 (2017).
- de Jong, W. et al. Correction to: iHIVARNA phase IIa, a randomized, placebo-controlled, double-blinded trial to evaluate the safety and immunogenicity of iHIVARNA-01 in chronically HIV-infected patients under stable combined antiretroviral therapy. Trials 20, 721 (2019).PubMed PubMed Central Article Google Scholar
- de Jong, W. et al. iHIVARNA phase IIa, a randomized, placebo-controlled, double-blinded trial to evaluate the safety and immunogenicity of iHIVARNA-01 in chronically HIV-infected patients under stable combined antiretroviral therapy. Trials 20, 361 (2019).PubMed PubMed Central Article CAS Google Scholar
- Miao, L. et al. Delivery of mRNA vaccines with heterocyclic lipids increases anti-tumor efficacy by STING-mediated immune cell activation. Nat. Biotechnol. 37, 1174–1185 (2019).CAS PubMed Article Google Scholar
- Gargano, J. W. et al. Use of mRNA COVID-19 vaccine after reports of myocarditis among vaccine recipients: update from the advisory committee on immunization practices—United States, June 2021. Morb. Mortal. Wkly Rep. 70, 977–982 (2021).CAS Article Google Scholar
- Mortezaee, K. Immune escape: a critical hallmark in solid tumors. Life Sci. 258, 118110 (2020).CAS PubMed Article Google Scholar
- Haibe, Y., El Husseini, Z., El Sayed, R. & Shamseddine, A. Resisting resistance to immune checkpoint therapy: a systematic review. Int. J. Mol. Sci. 21, 6176 (2020).CAS PubMed Central Article Google Scholar
- Yamamoto, T. N., Kishton, R. J. & Restifo, N. P. Developing neoantigen-targeted T cell-based treatments for solid tumors. Nat. Med. 25, 1488–1499 (2019).CAS PubMed Article Google Scholar
- Blass, E. & Ott, P. A. Advances in the development of personalized neoantigen-based therapeutic cancer vaccines. Nat. Rev. Clin. Oncol. 18, 215–229 (2021).PubMed PubMed Central Article Google Scholar
- Aurisicchio, L., Pallocca, M., Ciliberto, G. & Palombo, F. The perfect personalized cancer therapy: cancer vaccines against neoantigens. J. Exp. Clin. Cancer Res. 37, 86 (2018).PubMed PubMed Central Article CAS Google Scholar
- Zahm, C. D., Moseman, J. E., Delmastro, L. E. & Mcneel, D. G. PD-1 and LAG-3 blockade improve anti-tumor vaccine efficacy. Oncoimmunology 10, 1912892 (2021).PubMed PubMed Central Article Google Scholar
- BioNTech SE. United States Securities and Exchange Commission Form F-1 https://investors.biontech.de/node/8126/html (2020).
- Liang, X., Li, D., Leng, S. & Zhu, X. RNA-based pharmacotherapy for tumors: from bench to clinic and back. Biomed. Pharmacother. 125, 109997 (2020).CAS PubMed Article Google Scholar
- Wadhwa, A., Aljabbari, A., Lokras, A., Foged, C. & Thakur, A. Opportunities and challenges in the delivery of mRNA-based vaccines. Pharmaceutics 12, 102 (2020).CAS PubMed Central Article Google Scholar
- Giopanou, I. & Pintzas, A. RAS and BRAF in the foreground for non-small cell lung cancer and colorectal cancer: similarities and main differences for prognosis and therapies. Crit. Rev. Oncol. Hematol. 146, 102859 (2020).PubMed Article Google Scholar
- BioNTech. Next Generation Immunotherapy https://biontechse.gcs-web.com/static-files/b5a97ca9-efe5-485b-838c-940989ce8352 (2020).
- Sahin, U. et al. Mutanome Engineered RNA Immunotherapy (MERIT). Eur. Comm. Proj. Summ. https://www.liebertpub.com/doi/pdfplus/10.1089/humc.2015.2524 (2015).
- BioNTech. Securities and Exchange Commission Filing https://www.sec.gov/Archives/edgar/data/1776985/000119312520022991/d838504df1.htm (2020).
- Weide, B. et al. Results of the first phase I/II clinical vaccination trial with direct injection of mRNA. J. Immunother. 31, 180–188 (2008).CAS PubMed Article Google Scholar
- Rittig, S. M. et al. Intradermal vaccinations with RNA coding for TAA generate CD8+ and CD4+ immune responses and induce clinical benefit in vaccinated patients. Mol. Ther. 19, 990–999 (2011).CAS PubMed Article Google Scholar
- Weide, B. et al. Direct injection of protamine-protected mRNA: results of a phase 1/2 vaccination trial in metastatic melanoma patients. J. Immunother. 32, 498–507 (2009).CAS PubMed Article Google Scholar
- Kubler, H. et al. Self-adjuvanted mRNA vaccination in advanced prostate cancer patients: a first-in-man phase I/IIa study. J. Immunother. Cancer 3, 26 (2015).PubMed PubMed Central Article Google Scholar
- Rausch, S., Schwentner, C., Stenzl, A. & Bedke, J. mRNA vaccine CV9103 and CV9104 for the treatment of prostate cancer. Hum. Vaccin. Immunother. 10, 3146–3152 (2014).PubMed PubMed Central Article Google Scholar
- Sebastian, M. et al. A phase I/IIa study of the mRNA-based cancer immunotherapy CV9201 in patients with stage IIIB/IV non-small cell lung cancer. Cancer Immunol. Immunother. 68, 799–812 (2019).CAS PubMed Article Google Scholar
- Papachristofilou, A. et al. Phase Ib evaluation of a self-adjuvanted protamine formulated mRNA-based active cancer immunotherapy, BI1361849 (CV9202), combined with local radiation treatment in patients with stage IV non-small cell lung cancer. J. Immunother. Cancer 7, 38 (2019).PubMed PubMed Central Article Google Scholar
- Gandhi, L. et al. Phase 1/2 study of mRNA vaccine therapy + durvalumab (durva) ± tremelimumab (treme) in patients with metastatic non-small cell lung cancer (NSCLC). J. Clin. Oncol. 36, TPS9107 (2018).
- Sabari, J. et al. Phase 1/2 study of mRNA vaccine therapy + durvalumab (durva) ± tremelimumab (treme) in patients with metastatic non-small cell lung cancer (NSCLC). Cancer Immunol. Res. 7, B209 (2019).
- Wells, D. K. et al. Key parameters of tumor epitope immunogenicity revealed through a consortium approach improve neoantigen prediction. Cell 183, 818–834 (2020).CAS PubMed PubMed Central Article Google Scholar
- Esprit, A. et al. Neo-antigen mRNA vaccines. Vaccines 8, 776 (2020).
- Yarchoan, R. & Uldrick, T. S. HIV-associated cancers and related diseases. N. Engl. J. Med. 378, 1029–1041 (2018).PubMed PubMed Central Article Google Scholar
- Diken, M., Kranz, L. M., Kreiter, S. & Sahin, U. mRNA: a versatile molecule for cancer vaccines. Curr. Issues Mol. Biol. 22, 113–128 (2017).PubMed Article Google Scholar
- Sahin, U. et al. Personalized RNA mutanome vaccines mobilize poly-specific therapeutic immunity against cancer. Nature 547, 222–226 (2017).CAS PubMed Article Google Scholar
- Akinc, A. et al. Targeted delivery of RNAi therapeutics with endogenous and exogenous ligand-based mechanisms. Mol. Ther. 18, 1357–1364 (2010).CAS PubMed PubMed Central Article Google Scholar
- Cheng, Q. et al. Selective organ targeting (SORT) nanoparticles for tissue-specific mRNA delivery and CRISPR–Cas gene editing. Nat. Nanotechnol. 15, 313–320 (2020).CAS PubMed PubMed Central Article Google Scholar
- Kranz, L. M. et al. Systemic RNA delivery to dendritic cells exploits antiviral defence for cancer immunotherapy. Nature 534, 396–401 (2016).PubMed Article CAS Google Scholar
- Krohn-Grimberghe, M. et al. Nanoparticle-encapsulated siRNAs for gene silencing in the haematopoietic stem-cell niche. Nat. Biomed. Eng. 4, 1076–1089 (2020).CAS PubMed PubMed Central Article Google Scholar
- Moderna. Moderna Announces Positive Phase 1 Results for the First Systemic Messenger RNA Therapeutic Encoding a Secreted Protein (mRNA-1944) https://investors.modernatx.com/news-releases/news-release-details/moderna-announces-positive-phase-1-results-first-systemic (2019).
- CureVac. Genmab and CureVac Enter Strategic Partnership to Develop mRNA-based Antibody Therapeutics https://www.curevac.com/news/genmab-and-curevac-enter-strategic-partnership-to-develop-mrna-based-antibody-therapeutics (2019).
- Ethris. Neurimmune and Ethris Sign Collaboration Agreement to Rapidly Develop Inhaled mRNA-based Antibody Therapy for the Treatment of COVID-19 https://ethris.com/wp-content/uploads/2020/04/20200401_Neurimmune_and_Ethris_sign_collaboration_agreement_final.pdf (2020).
- Fernandez, A. M. A. et al. A phase I study (E011-MEL) of a TriMix-based mRNA immunotherapy (ECI-006) in resected melanoma patients: analysis of safety and immunogenicity. J. Clin. Oncol. 37, 2641 (2019).Article Google Scholar
- Hong, W. X. et al. Intratumoral immunotherapy for early-stage solid tumors. Clin. Cancer Res. 26, 3091–3099 (2020).CAS PubMed PubMed Central Article Google Scholar
- National Cancer Institute. CAR T Cells: Engineering Patients’ Immune Cells to Treat Their Cancers https://www.cancer.gov/about-cancer/treatment/research/car-t-cells (2019).
- Tchou, J. et al. Safety and efficacy of intratumoral injections of chimeric antigen receptor (CAR) T cells in metastatic breast cancer. Cancer Immunol. Res. 5, 1152–1161 (2017).CAS PubMed PubMed Central Article Google Scholar
- Maus, M. V. et al. T cells expressing chimeric antigen receptors can cause anaphylaxis in humans. Cancer Immunol. Res. 1, 26–31 (2013).CAS PubMed PubMed Central Article Google Scholar
- Beatty, G. L. et al. Activity of mesothelin-specific chimeric antigen receptor T cells against pancreatic carcinoma metastases in a phase 1 trial. Gastroenterology 155, 29–32 (2018).CAS PubMed Article Google Scholar
- Parayath, N. N., Stephan, S. B., Koehne, A. L., Nelson, P. S. & Stephan, M. T. In vitro-transcribed antigen receptor mRNA nanocarriers for transient expression in circulating T cells in vivo. Nat. Commun. 11, 6080 (2020).
- Zhang, F. et al. Genetic programming of macrophages to perform anti-tumor functions using targeted mRNA nanocarriers. Nat. Commun. 10, 3974 (2019).
- Fenton, O. S. et al. Synthesis and biological evaluation of ionizable lipid materials for the in vivo delivery of messenger RNA to B lymphocytes. Adv. Mater. 29, 1606944 (2017).
- McKinlay, C. J., Benner, N. L., Haabeth, O. A., Waymouth, R. M. & Wender, P. A. Enhanced mRNA delivery into lymphocytes enabled by lipid-varied libraries of charge-altering releasable transporters. Proc. Natl Acad. Sci. USA 115, E5859–E5866 (2018).PubMed PubMed Central Article CAS Google Scholar
- Lokugamage, M. P., Sago, C. D., Gan, Z., Krupczak, B. R. & Dahlman, J. E. Constrained nanoparticles deliver siRNA and sgRNA to T cells in vivo without targeting ligands. Adv. Mater. 31, 1902251 (2019).
- Zhao, X. et al. Imidazole‐based synthetic lipidoids for in vivo mRNA delivery into primary T lymphocytes. Angew. Chem. Int. Ed. Engl. 59, 20083–20089 (2020).CAS PubMed Article Google Scholar
- Tombácz, I. et al. Highly efficient CD4+ T cell targeting and genetic recombination using engineered CD4+ cell-homing mRNA–LNPs. Mol. Ther. 29, 3293–3304 (2021).PubMed Article CAS Google Scholar
- Gillmore, J. D. et al. CRISPR–Cas9 in vivo gene editing for transthyretin amyloidosis. N. Engl. J. Med. 385, 493–502 (2021).CAS PubMed Article Google Scholar
- Moderna. Moderna Reports Second Quarter Fiscal Year 2021 Financial Results and Provides Business Updates https://investors.modernatx.com/news/news-details/2021/Moderna-Reports-Second-Quarter-Fiscal-Year-2021-Financial-Results-and-Provides-Business-Updates-08-05-2021/default.aspx (2021).
- Moderna. Moderna Announces First Participant Dosed in Phase 1/2 Study of Its Quadrivalent Seasonal Flu mRNA Vaccine https://investors.modernatx.com/news-releases/news-release-details/moderna-announces-first-participant-dosed-phase-12-study-its (2021).
新冠之外mRNA三大用武之地:抗肿瘤疫苗、蛋白替代疗法、细胞疗法
5月9日,Nature Biotechnology杂志发表了题为“The clinical progress of mRNA vaccines and immunotherapies”的综述文章,回顾了mRNA疫苗及相关免疫疗法的临床进展。本篇将聚焦抗肿瘤mRNA疫苗及基于mRNA的蛋白免疫疗法与细胞免疫疗法。
抗肿瘤mRNA疫苗
肿瘤免疫疗法进展迅速,PD-1、CTLA-4、PD-L1、LAG-3等免疫检查点纷纷有药物获批,产业界研发热情高涨。将mRNA用于肿瘤免疫疗法的原理是利用mRNA编码突变肿瘤抑制蛋白,修饰肿瘤微环境,从而实现治疗目的。该方法的应用受限于mRNA递送技术。按照目前的递送能力,mRNA无法到达患者体内的每一个肿瘤细胞。因此,研发人员越来越关注将mRNA作为治疗性疫苗,诱导免疫系统识别并杀伤肿瘤细胞。
在新冠mRNA疫苗的研发中,产业界在快速开发和生产mRNA药物、体内编码疾病完整抗原方面获取的经验使得mRNA疫苗技术用于肿瘤治疗很有希望。此外,一些患者对目前的免疫靶向药物耐药,这也为mRNA疫苗用于肿瘤治疗创造了机会。
治疗性疫苗的开发过程有一系列常见的挑战。首先,传染病疫苗通过诱导机体体液免疫实现预防功能,而治疗性肿瘤疫苗还必须能够诱导强烈的CD8+T细胞反应以彻底根除所有肿瘤细胞。其次,开发肿瘤治疗性mRNA疫苗需要在体内编码能够诱导肿瘤高度特异性免疫反应的抗原。由于个体间抗原存在高度变异性,研究人员开发了一系列患者特异性的新抗原以应对这一挑战。最后,即便抗原能够诱导细胞免疫反应,抑制性肿瘤微环境可阻碍T细胞浸润肿瘤组织,甚至可能导致T细胞衰竭,因此治疗性疫苗还需要与克服抑制性肿瘤微环境药物(如免疫检查点抑制剂)联合使用。
1.肿瘤相关抗原
肿瘤相关抗原(TAA)主要分布于肿瘤细胞表面,是免疫系统攻击肿瘤的识别位点。肿瘤疫苗的靶标中包括了多种已知的TTA。
BNT111由编码4种黑色素瘤相关抗原[纽约食管鳞状细胞癌1(NY-ESO-1)、酪氨酸酶、黑色素瘤抗原A3(MAGE A3)和跨膜同源性磷酸酶-张力蛋白(TPTE)]的mRNA构成。4种TAA的mRNA序列均经过优化,可在未成熟的DC细胞中翻译出对应蛋白。每个序列还包含一段信号肽、破伤风类毒素CD4+P2和P6表位以及用于增强人类白细胞抗原( HLA )的抗原提呈和免疫原性功能的主要组织相容性复合体( MHC )Ⅰ转运结构域。
脾脏18-氟-2-脱氧-D-葡萄糖正电子放射断层造影术(PET)结果显示,脾脏代谢功能增强,表明淋巴组织激活。酶联免疫斑点分析结果表明,50例受试者中约75%对4种TAA中的至少1种IFN-γ有阳性结果,即产生免疫反应。抗原特异性T细胞为OD1+CCR7DD27+/D45RA效应记忆性T细胞表型,激活后可分泌IFN-γ和肿瘤坏死因子。在持续接种疫苗的患者中,TTA特异性细胞数量保持稳定甚至增加;而不再继续接种疫苗的患者中,T细胞可存活数月,后续逐渐下降。通过将从接种疫苗的患者处克隆的TAA特异性T细胞受体转染至健康供体CD8+T细胞中,转染后的细胞可以裂解黑色素瘤细胞系。
每次给药后,患者体内IFN-α、IFN-γ、IL-6和其他细胞因子水平提高,通常在接种后数小时达到峰值,24小时后恢复至正常水平,而这与观察到的不良反应事件发生特征一致。不良反应主要是轻度至中度的流感样症状,通常持续时间短暂且具有自限性(自限性疾病是指在疾病发生发展到一定程度后可以自动停止,并逐渐恢复痊愈的疾病,如水痘、玫瑰糠疹、斑秃和普通感冒等)。42例患者的影像学首次评估结果令人振奋。在25例接受单一疗法的受试者中,3例部分缓解,7例疾病稳定;而17例接受疫苗与PD-1联合疗法的患者中,6例部分缓解。有趣的是,2例曾接受抗PD-1治疗后疾病进展的患者,在接种疫苗后重新对PD-1疗法有响应,这一结果支持诱导T细胞属于PD1+效应记忆性T细胞表型的结论。目前,BNT111正在进行黑色素瘤的临床II期试验。
2.个性化新抗原
在肿瘤的发生发展过程中,恶性肿瘤细胞不断突变,产生正常细胞不表达的蛋白序列。这些蛋白质通过蛋白酶体加工成肽段,被T细胞识别。这些新抗原通常是每个患者独有的,为肿瘤特异性、患者定制的免疫疗法带来了机遇与挑战。
在设计编码患者特异性新抗原的mRNA疫苗时,需要采集患者的肿瘤样本,经过下一代测序技术鉴定患者特异的新抗原。编码这种新抗原的mRNA随即输注到该患者体内,以诱导免疫系统攻击肿瘤。然而,这一过程必须加快进度,要在肿瘤进一步进展之前对患者进行有效的治疗。据报道,上述步骤必须控制在30-40天。由于mRNA疫苗生产必须在GMP条件下进行,产品必须通过一定的质量标准,这种要求为药企带来了较大的挑战性。
迄今为止,个性化新抗原疫苗大部分工作仍集中于新抗原多肽疫苗,目前上述工作还未获得实质性成功。理论上讲,肿瘤突变负荷(TMB)最高的恶性肿瘤是新抗原疫苗的最佳应用场景,但也最有可能产生耐药性。与多肽疫苗相比,mRNA编码的新抗原免疫刺激性适中,可以提供更强的免疫原性,患者可获得更多临床获益。与多肽疫苗不同,mRNA可以编码整个抗原,呈现多个抗原表位。此外,mRNA疫苗可同时表达多个新抗原(多个mRNA分别表达不同的新抗原;或将不同的新抗原融合到同一mRNA序列中)。某些肿瘤可以产生几十种新抗原。基于诱导更广泛免疫反应的要求,表达多个表位更可能引发T细胞反应。
BioNTech已经开发了几种用于治疗肿瘤的新抗原候选疫苗。例如,BNT121已经在13例转移性黑色素瘤患者的腹股沟淋巴结中重复给药,临床结果令人鼓舞,该疫苗在患者体内诱发了强大的免疫反应。
BNT122(RO7198457)可编码多达20种个性化新抗原。初步结果表明,该药物单一疗法或与PD-1药物阿替利珠单抗(atezolizumab)联合使用都具有可接受的安全性,而不良反应主要是输液相关的反应和(或)细胞因子释放综合征(发热、寒颤等)。目前,BNT122正在进行黑色素瘤、非小细胞肺癌、结直肠癌的II期临床和三阴性乳腺癌的I期临床试验。
蛋白免疫疗法与细胞免疫疗法
mRNA的一个新兴领域是体内编码可用于治疗的免疫蛋白或免疫调节蛋白,如抗体与细胞因子。与传染病疫苗、肿瘤疫苗相比,这些疗法需要产生更多的蛋白才可发挥作用,某些疾病甚至需要终身服药。
蛋白免疫疗法的一大挑战是将mRNA递送到所有目标器官和细胞中,实现最佳的治疗效果。例如,某些蛋白需要进一步PTM(如糖基化与蛋白水解)才能充分发挥功能。而PTM对蛋白的修饰作用可能是组织依赖性的,而不仅仅取决于mRNA的序列,这进一步说明mRNA需要特异性递送至目标组织。
当mRNA以LNP为载体全身给药时,由于载脂蛋白E可结合至LNP表面,LNP复合物将倾向于分布到肝脏组织,肝细胞表面受体将诱导LNP颗粒的肝细胞摄取。通过调整LNP中脂质成分(包括调整脂质比例和组成)可实现非肝脏的特异性组织分布,如肺组织内皮细胞或脾脏的靶向递送。最近,通过调节PEG-脂质结构,改变LNP表面性质,实现了LNP特异性靶向造血干细胞生态龛中的骨髓内皮细胞。
因此,与疫苗相比,mRNA蛋白免疫疗法在制剂递送、蛋白生产有效性和耐受性方面提出了新的挑战。这也可能是mRNA治疗药物比mRNA疫苗开发进度慢的原因。
1.mRNA编码单抗疗法
直接将mRNA递送至特定组织或器官是mRNA药物开发的屏障。只要药物是安全的,同时合理设计给药剂量控制蛋白表达水平,mRNA药物全身给药是一种合适的给药途径。mRNA-1944是一种编码单克隆抗体(mAb)的mRNA药物。这种药物以LNP为载体,编码一种识别基孔肯雅病毒(Chikungunya virus)的mAb。首项基于健康受试者的临床试验数据表明,所有试验剂量(0.1,0.3,0.6 mg/kg)中都可以检测到中和抗体。但在最高剂量组下,4例受试者中3例出现输注相关反应,其中1例受试者出现3级心动过速、白细胞计数升高、2级恶心、呕吐、发热,同时心电图上存在短暂倒置T波。而经类固醇预处理的同一剂量组别下的另一组受试者没有出现3级不良反应,但基孔肯雅病毒特异性抗体水平(Emax)降低了1.7倍。
以mRNA产生抗体吸引了很多关注,目前业内有多项该领域的合作正在进行。例如,CureVac正和Cenmab合作开展一项基于mRNA的mAb抗肿瘤疗法;Neurimmune和Ethirs合作开展了吸入式mRNA药物,编码抗新冠mAb。这里需要重点考虑的因素是,为什么采用mRNA体内编码抗体,而不是利用传统的重组生产工艺?最有潜力的方法需要综合考虑给药剂量、持续时间、PTM类型、递送系统和目标抗体的安全性等因素。
2.mRNA编码免疫刺激蛋白
另一种抗肿瘤的方法是注射可编码具有直接治疗效果蛋白的mRNA,编码蛋白通过刺激免疫系统[如OX40配体(OX40L)或IL]实现肿瘤杀伤作用。例如,mRNA-2416是一种可编码免疫检查点调节分子OX40L的mRNA药物。在一项临床研究中,41例罹患多种恶性肿瘤的受试者瘤内单药给药没有达到《实体瘤疗效评价标准》(Response Evaluation Criteria in Solid Tumors)中规定的部分缓解的标准。目前,申办方计划进行一项与度伐利尤单抗联合给药用于治疗卵巢癌的临床II期拓展性队列研究。
编码多种不同免疫调节剂的mRNA药物ECI-006是TriMix[编码激活DC细胞多种分子(CD40L、CD70和caTLR4)的mRNA]和编码黑色素瘤特异性TAA(酪氨酸、gp100、MAGE A3、MAGEC2和PRAME的mRNA)的组合。这种药物药物正在切除黑色素瘤的患者中进行临床I期试验。
另一种思路是产生免疫调节融合蛋白。MEDI1191编码含有IL-12α、IL-12β亚基的单链融合蛋白,两条亚基间以一条连接基团结合。这种药物瘤内注射,预期可以提高全身给药对重组IL-12的耐受性。
3.过继免疫细胞疗法中的mRNA
过继性免疫疗法是一种相对较新的治疗方法,这种方法从患者体内采集自身免疫细胞,体外处理后回输到患者体内,达到治疗肿瘤的目的。Tchou等的研究表明,在乳腺癌瘤内注射转染了编码靶向c-Met的嵌合抗原受体(CAR)mRNA的T细胞,耐受性良好,可以在肿瘤组织中诱发免疫反应。
纳米制剂帮助mRNA递送至不同的免疫细胞,如巨噬细胞、B细胞和T细胞等,进一步扩大了免疫疗法的应用前景。例如, T细胞靶向递送mRNA技术为体内生成CAR-T细胞创造了可能性,开辟了肿瘤治疗的新方式。
mRNA疗法的优势与改进空间
1、优势
mRNA在基因疗法和蛋白疗法中占据了独特的地位,结合了两种疗法的优势,又规避了二者面临的挑战。例如,在生物反应器中生产多聚体蛋白难度很大,而这一挑战可以通过单个mRNA或多种mRNA编码蛋白不同亚基后,在患者体内自组装解决。
mRNA在一种CMV候选疫苗和多种肿瘤领域的应用证明了这一技术的灵活性。当前形势下,世界正面临着新冠疫情的威胁,新的毒株层出不穷。这些毒株对已获批的新冠疫苗具有不同的敏感性。随着该领域技术的不断优化和完善,未来还有可能开发出针对传染病和肿瘤以外适应症的mRNA药物。
编码蛋白表达的短暂性使mRNA疗法成为需要单个或少数几种蛋白表达条件下的理想方式,如传染病疫苗。给药可重复、剂量可调整、给药间隔可选择的特性让这种技术具有和经典药物治疗一样的灵活性,使其成为一个可适应患者个体需求的选择,减轻患者对这种新技术的疑虑。而从安全性的角度,两项临床试验(mRNA-1273和mRNA-1647)结果表明,第2次接种后产生的不良反应比第一次接种后更加严重,但这不是一个普遍现象。一些患者接种了超过8次BNT111疫苗,没有观察到严重的不良反应。吸入性mRNA药物MRT5005每周最多服用5次,在此期间没有观察到安全性恶化的趋势。
通过对重组蛋白的研究,mRNA疗法的潜力将进一步扩大。例如,mRNA可编码Fc结构域与治疗结构域的融合蛋白,可有效延长蛋白质的体内半衰期。而更令人兴奋的是将mRNA用于细胞疗法。mRNA基因编辑的瞬时作用可以避免永久表达带来的不良反应。此外,细胞内表达抗体、抗体片段或其他蛋白结合基序可在特定的细胞器(如细胞核)表达,以充分发挥编码蛋白的功效。
mRNA目前还只是作用于免疫系统,应用场景包括传染病疫苗与肿瘤疫苗。新冠mRNA疫苗的获批上市为mRNA技术的可行性验证提供了前所未有的机会,而目前肿瘤疫苗还没有新药获批上市。BNT111的结果表明,将充足的蛋白表达与免疫激活通路相结合可以解决早前蛋白疫苗遇到的一些困难。
2、改进空间
由于RNA可以通过TLR和RIG-1信号通路激活免疫系统,RNA的免疫刺激性对mRNA药物具有非常重要的意义。例如,CV8102没有编码能力,而是作用免疫佐剂。这种免疫刺激性的缺点在于可能引发某些mRNA药物的安全性与耐受性问题。越来越多的证据表明,mRNA药物最常见的副作用是某些炎症反应。例如,在肌内注射或皮下注射时会出现疼痛、红肿、酸胀的局部反应;在肌内注射或吸入药物时可能出现发热综合征或流感样反应。这些症状都可以用抗炎药处理。在静脉注射编码基孔肯雅病毒mAb的mRNA的临床试验中,接受最高剂量的受试者中预先采用类固醇处理可以降低不良反应的发生率,但是这种处理方法降低了编码蛋白的表达水平。
这些数据为未来进一步研究mRNA重复给药的发展方向提供了一些线索。例如,类固醇对缓解mRNA诱发的炎症反应是否有效和必需?如果答案是肯定的,这是否意味着要以牺牲编码蛋白的表达水平为代价?而副作用减少和编码蛋白水平降低是否意味着一定程度的炎症反应实际上是充分的编码蛋白表达的先决条件?如果上述问题的答案都是肯定的,那么临床实践中必须平衡炎症反应程度与编码蛋白表达水平,不能产生阻碍重复给药的不良反应。
回答这些问题并不简单,因为大多数mRNA药物并不是直接注射裸mRNA,而是将其包裹在LNP或PNP中,提高制剂的耐受性。后续的临床试验中,对照组中采用不含mRNA的空白LNP有助于回答mRNA和LNP对制剂耐受性的作用。但是这种对照也有一个缺陷,即空LNP不与带负电荷的mRNA结合时,其物理化学性质与装载有mRNA的LNP制剂不同,不能称为一个严格的对照组。
尽管在单次疫苗接种和用于治疗肿瘤等危及生命的应用场景下,短暂的炎症反应是可以接受的,但对于需要长期治疗的适应症,尤其是通过静脉注射的给药方式,选择具有良好耐受性和安全性的脂质和其他辅料至关重要。动物实验数据在这方面的价值有限,因为报道指出,BNT111在人类中触发的细胞因子浓度比在小鼠体内低1000倍以上;而MRT5005的动物实验中未观察到人体试验中发生的发热反应。
除即刻耐受性外,脂质蓄积带来的长期问题也需要考虑。若mRNA编码的蛋白质半衰期较短,则需要缩短给药间隔以维持临床疗效所必须的蛋白表达量,这可能引发脂质在靶组织和非靶组织中的蓄积,带来健康风险。mRNA的处方工艺将与mRNA生物学一道成为未来研发的重点。
mRNA的给药途径也很有想象空间,如肌内给药、皮内给药、皮下给药、淋巴结注射、瘤内注射、静脉注射和吸入给药等。经过进一步发展,未来有望实现鼻内疫苗、滴眼液或滴鼻液、皮肤药膏、肛门栓剂、膀胱灌注溶液、鞘内输注或Ommaya囊等。未来mRNA的治疗领域将直接取决于纳米药物递送技术的进步。越来越多的证据表明,LNP和PNP可以实现机体组织靶向(如肝脏、内皮、肺、骨骼以及免疫系统的多个组织)。而通过对递送材料的改进以及其他功能性材料的修饰,提高递送系统的靶向性与递送能力,mRNA的应用场景将进一步拓宽。
目前,两款经EUA获批的新冠疫苗凸显了mRNA的一大优势,即临床试验药物的快速生产能力。最初为个体化新抗原疫苗开发的制备工艺使得新冠病毒序列公布后的数周,候选mRNA疫苗临床试验随即展开,充分证明了其快速响应能力。
然而,新冠疫苗的使用也凸显了这项技术的另一个问题:对冷链贮存与运输的依赖。mRNA需要在-80℃贮存与运输,不是所有药房和临床试验基地都具备这样的条件;对于患者在家中自行给药,-20℃的保存条件都可能难以满足。因此,提高mRNA药物的稳定性将是其处方工艺研究的下一个重点。
小结
总结来说,在新冠疫苗的竞赛中,mRNA作为一种安全、有效的技术平台,加速了全球对于mRNA-LNP系统风险/获益的认知。目前,公众对于BNT162b2和mRNA-1273的生产能力、储存条件以及两种疫苗的副作用更加关注;而学界更加关注这两种疫苗之外的问题,迫切地希望mRNA能够在其他适应症中得以验证。
参考资料:[1] Ann J. Barbier et al. The clinical progress of mRNA vaccines and immunotherapies. Nature Biotechnology. 2022.[2] 李颖. 细胞抗病毒天然免疫信号转导的调控机制. 武汉大学出版社. 2015.[3] 魏跃钢,王晓华. 皮肤病治疗与调养. 人民军医出版社. 2015.