Verve Therapeutics Announces Interim Data for VERVE-101 Demonstrating First Human Proof-of-Concept for In Vivo Base Editing with Dose-Dependent Reductions in LDL-C and Blood PCSK9 Protein in Patients with Heterozygous Familial Hypercholesterolemia
来源:verve/药明康德 | 发布时间:2023-11-15
LDL-C Reductions Up to 55% and Blood PCSK9 Protein Reductions Up to 84% Observed After a Single Infusion of VERVE-101 at Potentially Therapeutic Doses
Safety Profile Supports Continued Development of VERVE-101
Enrollment Ongoing in the 0.45 mg/kg and 0.6 mg/kg Cohorts with Plans to Initiate Expansion Cohort in 2024
Company to Host Conference Call and Webcast Today at 6:30 p.m. ET
BOSTON, Nov. 12, 2023 (GLOBE NEWSWIRE) — Verve Therapeutics, Inc., a clinical-stage biotechnology company pioneering a new approach to the care of cardiovascular disease with single-course gene editing medicines, today announced first human proof-of-concept data for in vivo base editing from the ongoing heart-1 phase 1b clinical trial of VERVE-101. Treatment with VERVE-101 led to dose-dependent reductions of disease-causing low-density lipoprotein cholesterol (LDL-C) in people living with heterozygous familial hypercholesterolemia (HeFH), a life-threatening inherited disease characterized by lifelong elevations in blood LDL-C and accelerated atherosclerotic cardiovascular disease (ASCVD). VERVE-101 is an investigational, in vivo base editing medicine designed to be a single-course treatment that inactivates the PCSK9 gene in the liver to durably lower blood LDL-C.
“Of the more than three million people with HeFH in the U.S. and Europe, very few are currently at LDL-C goal, due in part to a care model that requires lifetime therapies. This model puts a strain on the healthcare system and is failing our patients,” said Deepak L. Bhatt, M.D., M.P.H., Director of the Mount Sinai Fuster Heart Hospital and the Dr. Valentin Fuster Professor of Cardiovascular Medicine at the Icahn School of Medicine in New York. “I am very encouraged by the initial data from the heart-1 trial that demonstrated the potential for single-course gene editing as a new approach to treat patients with HeFH. The data showed that VERVE-101 could meaningfully and durably lower LDL-C in these patients. This trial enrolled patients with advanced coronary disease, and the cardiovascular adverse events were consistent with what might be expected in this patient population. We’re at an exciting moment for cardiovascular prevention where the management of ASCVD may fundamentally change.”
heart-1 Clinical Trial Design
heart-1 is an open-label, phase 1b clinical trial in patients living with HeFH, established ASCVD and uncontrolled hypercholesterolemia. The trial is designed to evaluate the safety and tolerability of VERVE-101, with additional analyses for pharmacokinetics and pharmacodynamic reductions in blood PCSK9 protein and LDL-C. Single doses of 0.1 mg/kg (n=3), 0.3 mg/kg (n=3), 0.45 mg/kg (n=3), and 0.6 mg/kg (n=1) of VERVE-101 have been administered via intravenous infusion. Initial safety data reported are from all ten patients enrolled as of a data cut-off date of October 16, 2023. One patient who received a 0.45 mg/kg dose had not reached day 28 as of the data cut-off date and is not included in the efficacy analysis.
Patients included in both the safety and efficacy analyses have had a high burden of coronary artery disease, consistent with the 2022 U.S. Food and Drug Administration (FDA) draft guidance for human genome editing products1 that suggests a first-in-human trial include patients with severe, advanced disease. Nine patients have had prior coronary revascularizations with either coronary artery bypass grafting or coronary stenting procedures and four have had prior myocardial infarctions. With a mean screening LDL-C of 193 mg/dL, none of the patients were at LDL-C goal on maximally tolerated oral lipid-lowering therapy.
heart-1 Efficacy Analysis
Following a single infusion of VERVE-101, dose-dependent reductions in pharmacodynamic measures of blood PCSK9 protein levels were observed, suggesting successful editing at the intended genomic target. Dose-dependent LDL-C reductions, a validated measure of clinical efficacy for this patient population, were observed one month after treatment.
In the interim dataset, six patients were treated at sub-therapeutic doses (0.1 mg/kg and 0.3 mg/kg) and three patients were treated at potentially therapeutic doses (0.45 mg/kg and 0.6 mg/kg). The two patients treated with 0.45 mg/kg of VERVE-101 had a time-averaged blood PCSK9 protein reduction of 59% and 84%. The patient treated with 0.6 mg/kg of VERVE-101 had a time-averaged blood PCSK9 protein reduction of 47%.
The two patients treated with 0.45 mg/kg of VERVE-101 had a time-averaged LDL-C reduction of 39% and 48%. The patient treated with 0.6 mg/kg of VERVE-101 had a time-averaged LDL-C reduction of 55%. In this single participant in the highest dose cohort, the 55% reduction in LDL-C was durable out to 180 days, with follow-up ongoing.
Blood PCSK9 protein and LDL-C reductions are quantified as percent change from baseline using the time-weighted average from day 28 through last available follow-up.
heart-1 Safety and Tolerability
The safety profile observed in the heart-1 trial supports continued development of VERVE-101, and the adverse events have been consistent with the severe, advanced ASCVD patient population enrolled.
VERVE-101 was well-tolerated in the two lower dose cohorts, with no treatment-related adverse events observed. In the two higher dose cohorts, treatment-related adverse events were observed, including transient, mild or moderate infusion reactions and transient, asymptomatic increases in liver transaminases with mean bilirubin levels below the upper limit of normal. All infusion reactions and liver transaminase elevations resolved without clinical sequelae.
Two patients experienced serious adverse events, which were each cardiovascular events in the context of severe underlying ASCVD. One patient dosed in the 0.3 mg/kg cohort had a fatal cardiac arrest approximately five weeks after treatment due to underlying ischemic heart disease, which was determined by the investigator and independent data and safety monitoring board (DSMB) to be not related to treatment.
One patient dosed in the 0.45 mg/kg cohort experienced a myocardial infarction (Grade 3) the day after treatment. The event was considered potentially related to treatment due to the proximity to dosing. The event occurred in the setting of unstable chest pain symptoms prior to dosing that were unreported to investigators. Coronary angiography taken after the event showed critical left main equivalent coronary artery disease. The same patient also experienced non-sustained ventricular tachycardia (Grade 2) more than four weeks after dosing, which was determined to be unrelated to treatment.
All safety events were reviewed with the independent DSMB who recommended continuation of trial enrollment with no protocol changes required.
“We are excited to have reached this milestone of positive first-in-human data supporting the significant potential for in vivo liver gene editing as a treatment for patients with HeFH. VERVE-101 is the first in vivo base editor to be evaluated in the clinic,” said Sekar Kathiresan, M.D., co-founder and chief executive officer of Verve. “This milestone is only possible because of the incredible patients, families, and physicians who are participating in our study, and the highly talented team at Verve in their steadfast commitment to bringing VERVE-101 forward.”
“Our goal is to fundamentally disrupt the chronic care model for cardiovascular disease and provide a new single-course treatment option for patients,” said Andrew Bellinger, M.D., Ph.D., chief scientific officer of Verve. “These data confirm our hypothesis that a single-course gene editing medicine has the potential to induce meaningful and durable reductions in LDL-C when administered at therapeutic doses. Based on the favorable initial findings in the heart-1 trial, we are continuing to enroll patients in the potentially therapeutic dose cohorts. And with the recent clearance of the U.S. investigational new drug (IND) application for VERVE-101, we look forward to expanding our clinical trial into the U.S.”
Next Steps
The heart-1 trial is enrolling patients in the 0.45 mg/kg and 0.6 mg/kg cohorts in the United Kingdom and New Zealand. With the recent clearance of the IND application by the FDA for VERVE-101, Verve plans to activate and open U.S. sites. In 2024, the company plans to select a single dose from the dose escalation phase, initiate an expansion cohort, and complete this expansion cohort of the heart-1 clinical trial. In the first half of 2024, the company plans to initiate a phase 1 clinical trial of VERVE-102, subject to regulatory clearance. VERVE-102 is an in vivo base editing medicine that aims to inactivate the PCSK9 gene in a similar way to VERVE-101. VERVE-101 and VERVE-102 share an identical guide RNA targeting PCSK9 as well as similar messenger RNA expressing an adenine base editor; however, VERVE-102 is delivered using the company’s proprietary GalNAc-LNP delivery technology. Following completion of the heart-1 trial and the VERVE-102 trial, Verve plans to initiate a randomized, placebo-controlled phase 2 clinical trial of either VERVE-101 or VERVE-102 in 2025.
Conference Call Information
Verve will host a webcast investor event today, November 12 at 6:30 p.m. ET to review the heart-1 clinical trial data. The event can be accessed under Events in the Investors section of the company’s website at www.VerveTx.com. The archived webcast will be available on the company’s website beginning approximately two hours after the event.
About heart-1 and HeFH
heart-1 is an open-label phase 1b clinical trial designed to enroll adult patients with heterozygous familial hypercholesterolemia (HeFH) who have established atherosclerotic cardiovascular disease (ASCVD) to evaluate the safety and tolerability of VERVE-101 administration, with additional analyses for pharmacokinetics and reductions in blood PCSK9 protein and low-density lipoprotein cholesterol (LDL-C).
HeFH is a prevalent and potentially life-threatening subtype of ASCVD. High cumulative life-long exposure to LDL-C drives the development of atherosclerotic plaque that results in the hardening of arteries seen in ASCVD. The relationship between lowering of cumulative LDL-C exposure and reduction in the risk of ASCVD is among the best understood relationships in medicine.
About Verve Therapeutics
Verve Therapeutics, Inc. (Nasdaq: VERV) is a clinical-stage genetic medicines company pioneering a new approach to the care of cardiovascular disease, potentially transforming treatment from chronic management to single-course gene editing medicines. The company’s initial three programs – VERVE-101, VERVE-102, and VERVE-201 – target genes that have been extensively validated as pharmacologic targets for lowering low-density lipoprotein cholesterol (LDL-C), a root cause of cardiovascular disease. VERVE-101 and VERVE-102 are designed to permanently turn off the PCSK9 gene in the liver and are being developed initially for heterozygous familial hypercholesterolemia (HeFH) and ultimately to treat atherosclerotic cardiovascular disease (ASCVD) patients not at LDL-C goal on oral therapy. VERVE-201 is designed to permanently turn off the ANGPTL3 gene in the liver and is initially being developed for homozygous familial hypercholesterolemia (HoFH) and ultimately to treat patients with refractory hypercholesterolemia. For more information, please visit www.VerveTx.com.
一次性永久降低心血管疾病风险,基因编辑疗法首获人体概念验证
近期,Verve Therapeutics首次公布了其单碱基编辑疗法VERVE-101,在正在进行的1b期临床试验heart-1中获得的人体概念验证数据。数据显示,这种创新疗法在家族性高胆固醇血症(HeFH)患者中显示出剂量依赖性降低有害低密度脂蛋白胆固醇(LDL-C)的趋势。最高剂量可以一次治疗,将患者LDL-C水平降低55%,并持续长达180天以上。
心血管疾病是全球导致死亡的首要原因。然而尽管医学上已有充分数据证明,很多心血管疾病可以预防,然而真正做到防患于未然却并不容易。饮食控制和锻炼虽然能够有效降低LDL-C水平,但是大多数人无法持久地严格遵循这种生活方式。虽然已经有口服的他汀类药物,但用药依从性是心血管疾病防治的另一重大挑战。
Verve Therapeutics首席执行官,同时也是心脏病专家和科学家的Sekar Kathiresan博士在2022药明康德健康产业论坛上指出:“如果你问今天100名心脏病发作的患者,一年后还有多少人仍在服用降胆固醇药物,这个数字只有不到50%。”
Verve公司的VERVE-101利用基于CRISPR系统改造的单碱基编辑器,改变患者细胞中PCSK9基因的一个字母,达到让PCSK9永久失活的效果。PCSK9是降低LDL-C的热门靶点,抑制它活性的功效已经得到了多款FDA批准疗法的验证。
初步临床试验数据表明,单次注射VERVE-101可导致血液PCSK9蛋白水平的剂量依赖性降低,证实了成功的基因组编辑。相应在治疗后一个月观察到了LDL-C的剂量依赖性下降,这是这一患者群体的关键疗效指标。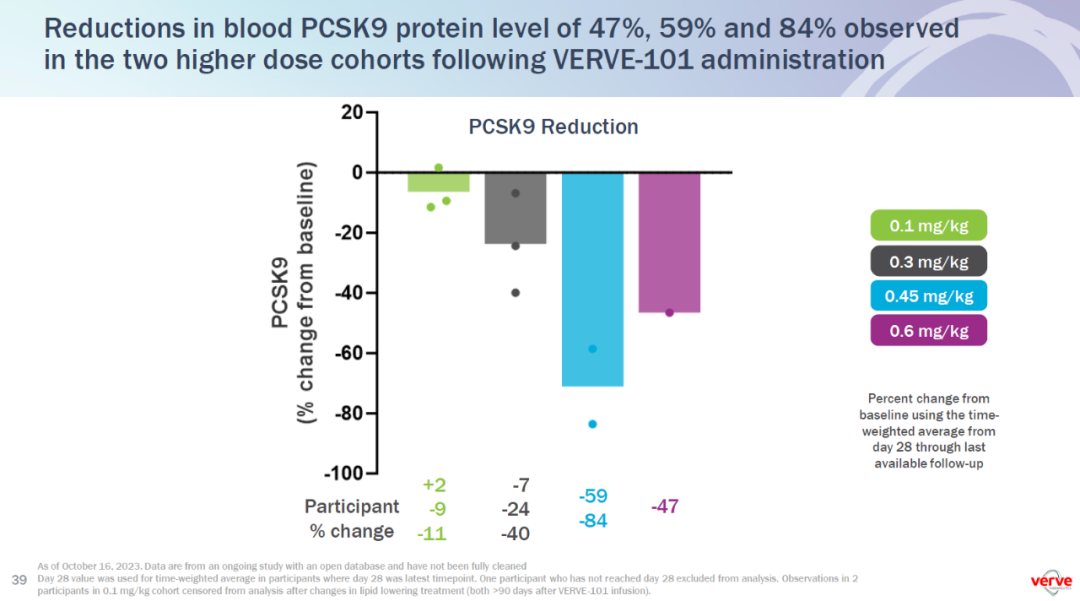
▲VERVE-101在1b期临床试验中剂量依赖性降低PCSK9蛋白水平(图片来源:Verve公司官网)
在当前数据集中,6名患者接受了低于治疗剂量的治疗(0.1 mg/kg和0.3 mg/kg),3名患者接受了可能具有治疗效果的剂量(0.45 mg/kg和0.6 mg/kg)。值得注意的是:
- 接受0.45 mg/kg剂量治疗的患者,其平均血液PCSK9蛋白水平分别降低了59%和84%。
- 接受0.6 mg/kg剂量治疗的患者,其平均血液PCSK9蛋白水平降低了47%。
- 在0.45 mg/kg组中观察到了39%和48%的LDL-C降低,而在接受0.6 mg/kg治疗的患者中,LDL-C降低了55%,持续时间长达180天,并且随访仍在进行中。
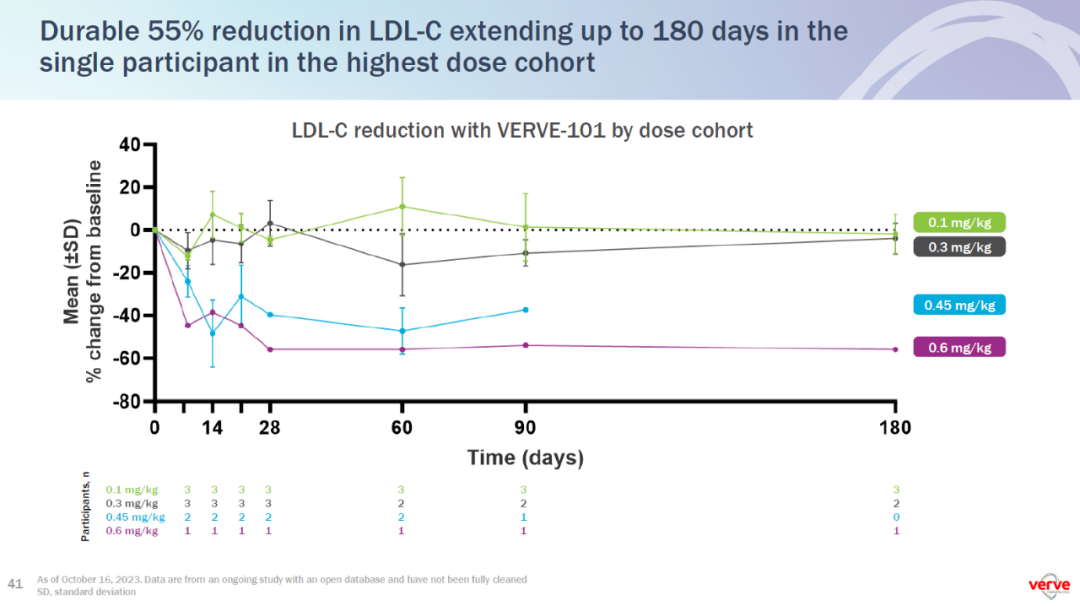
▲VERVE-101在1b期临床试验中剂量依赖性降低LDL-C蛋白水平(图片来源:Verve公司官网)
Heart-1试验的安全性数据支持VERVE-101的持续开发。观察到的不良事件与预期的严重晚期动脉粥样硬化心血管疾病患者群体一致。
在两个较低剂量队列中,VERVE-101表现出良好的耐受性,未观察到与治疗相关的不良事件。然而,在较高剂量队列中,观察到轻度至中度的短暂输液反应和无症状的肝脏转氨酶升高。这些副作用未引起临床后果而且自行消除。
两名患者发生了严重的不良心血管事件,分别在0.3 mg/kg和0.45 mg/kg队列中。独立的数据监察委员会在审查后得出结论,认为这些事件与治疗无直接关系。
目前,Verve正在英国和新西兰进行0.45 mg/kg和0.6 mg/kg队列的招募。在FDA许可VERVE-101的IND申请后,Verve计划启动美国临床试验点。2024年,该公司计划选择最佳剂量,扩大患者队列,并完成heart-1试验。此外,计划在2024年上半年开始VERVE-102的1期临床试验,这是另一种有前景的体内基因编辑药物。VERVE-101和VERVE-102都使用相同的PCSK9靶向碱基编辑器,但VERVE-102采用了Verve专有的GalNAc-LNP递送技术。
Kathiresan博士在2021药明康德全球论坛中曾表示,在过去的20年里,我们一直专注于研究和解读与疾病风险相关的基因组,未来20年人类将会为了健康而改写基因组。