The next generation of coronavirus vaccines: a graphical guide
来源:Nature | 发布时间:2023-02-06
New technologies might provide more potent or broader immunity — but will have to fight for market share.
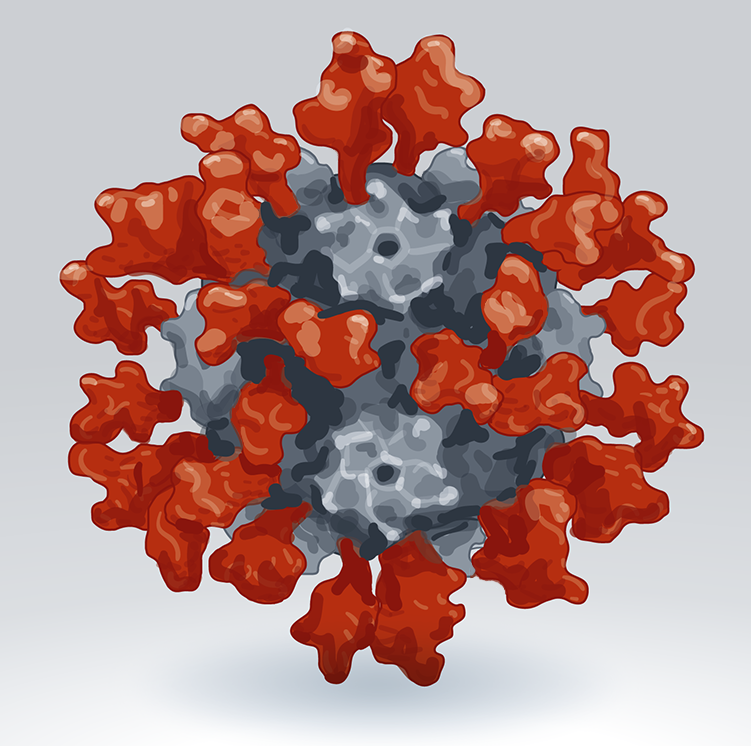
Vaccines against the coronavirus SARS-CoV-2 have been given to billions of people to protect them from COVID-19, and have saved more than 20 million lives. But viral variants can evade some of the immunity provided by the original vaccines. As a result, vaccine developers around the world are working on dozens of ‘next-generation’ COVID-19 vaccines: not just updates of the first versions, but ones that use new technologies and platforms.
These vaccines are a diverse group, but the overarching aim is to deliver long-lasting protection that is resilient to viral change. Some could protect against broader classes of coronavirus, including ones that have yet to emerge. Others might provide more potent immunity, might do so at lower doses, or might be better at preventing infection or transmission of the virus.
Here’s what to expect of this next generation of vaccines.
Why do we need more vaccines?
In a word: evolution. The first approved COVID-19 vaccines were tested for protection against versions of SARS-CoV-2 that had not changed much since the virus was first identified. These vaccines come in different types — some are composed of messenger RNA, others are inactivated versions of the coronavirus itself or some of its proteins — but all work by exposing the body to antigens (portions of the virus) to provoke an immune response without causing disease.
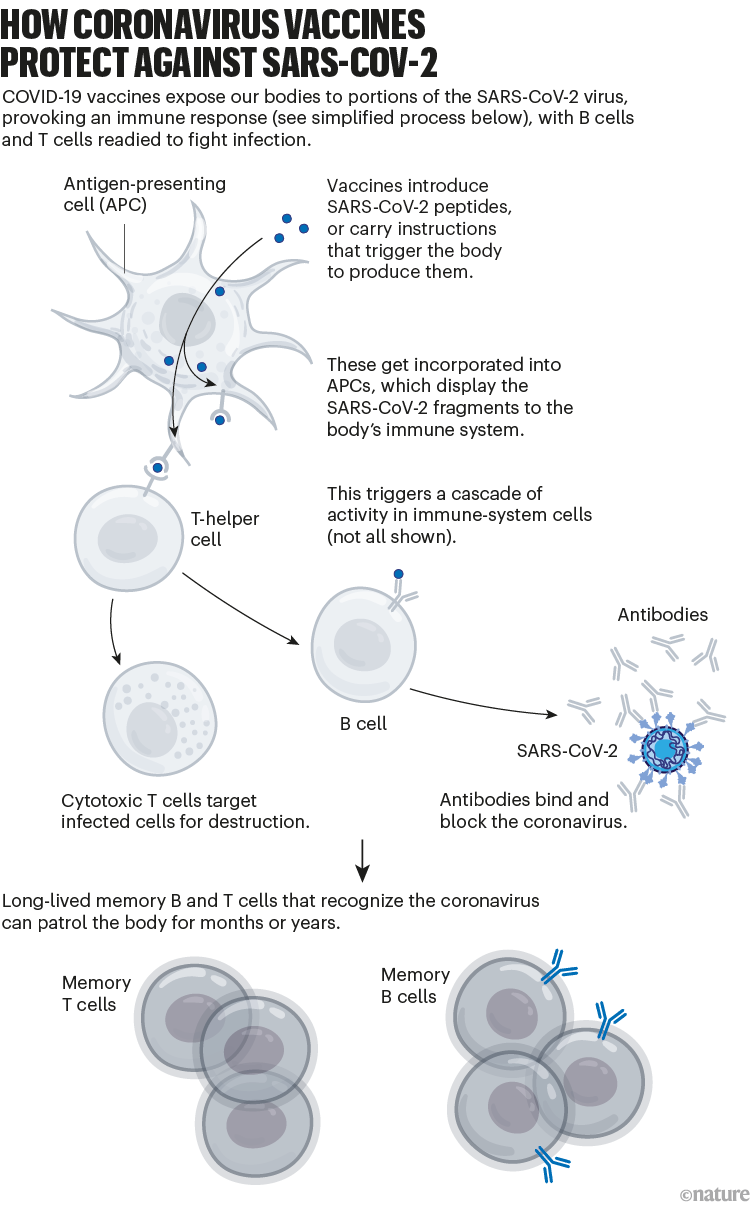
Broadly speaking, this immune response comes from B cells, which produce antibodies that can block SARS-CoV-2 from infecting cells, and from T cells, which can destroy infected cells (and support other immune responses).
The vaccinations also generate a pool of ‘memory cells’ for prolonged immunity, even after initial antibody levels dwindle. On subsequent infection, memory B cells begin proliferating and differentiating into cells that churn out more antibodies (see ‘How coronavirus vaccines protect against SARS-CoV-2’).
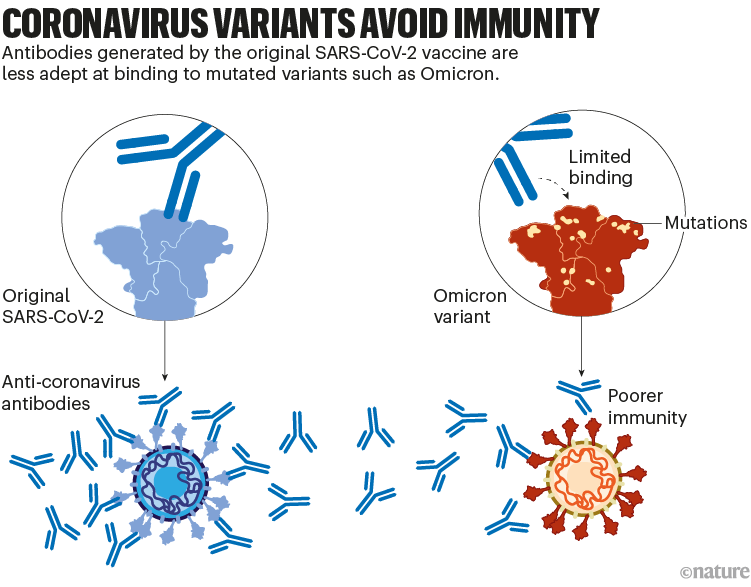
Although these vaccines provide long-lasting protection against severe disease, the protection they offer against viral infection dwindles in months. And variants of SARS-CoV-2, such as Omicron, have since evolved with mutations that allow them to escape some of this immunity. For instance, memory responses generated by the initial vaccines produce antibodies that don’t latch on to Omicron as easily. That contributes to the reduced protection against infection (see ‘Coronavirus variants avoid immunity’).
A second generation of vaccines has already been introduced to boost immunity against the Omicron variant. It’s likely that further, variant-specific updates to vaccines will follow, to try to keep up with viral evolution — although it’s not clear whether the protection they offer will be particularly long-lasting as immunity wanes and SARS-CoV-2 evolves further.
As a result, research teams are taking several approaches to develop new vaccines.
Updated vaccines
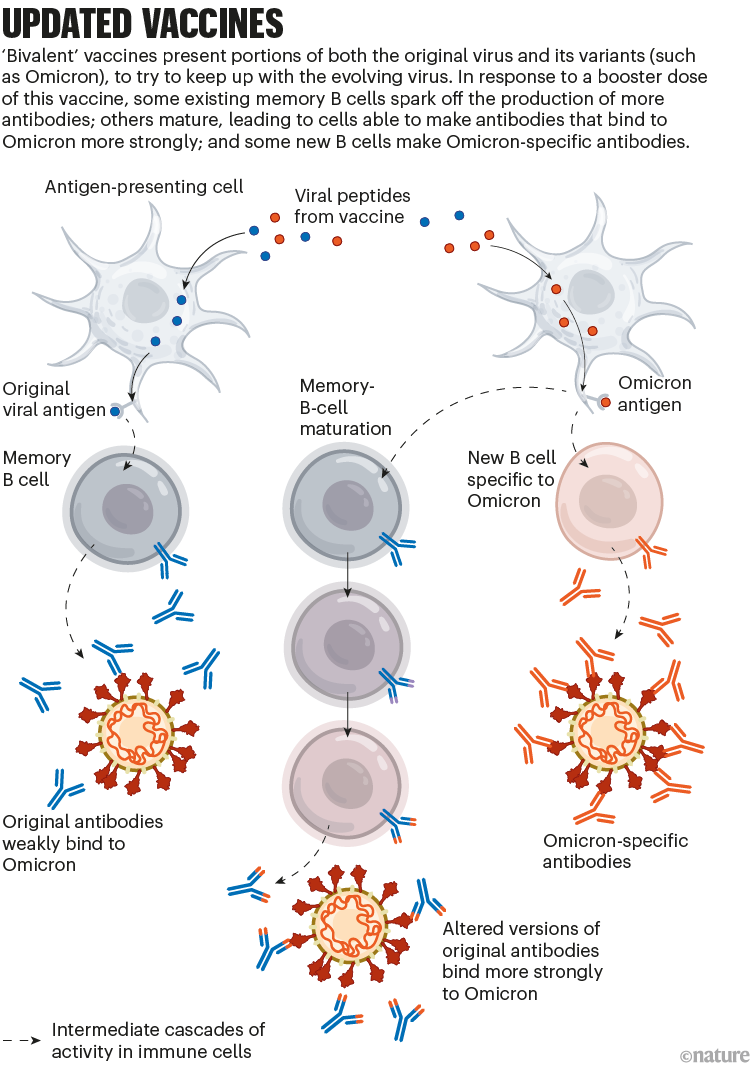
To tackle SARS-CoV-2 variants, the vaccine developers Pfizer–BioNTech and Moderna introduced updated mRNA vaccines last year. These are called bivalent, because they encode molecules of the spike protein from the original virus and from Omicron. (The spike protein is what SARS-CoV-2 uses to bind to cells.)
The bivalent vaccines work in several ways. Like other COVID-19 booster shots, they stimulate the memory B cells already established by previous vaccines; some of this cellular response leads to antibodies that can recognize Omicron. Their potency can strengthen over time, too: when presented with Omicron’s spike, memory B cells go through an evolutionary ‘training’ process of mutation and selection, producing a pool of B cells that encode antibodies that bind more tightly to Omicron’s spike. Finally, the Omicron components of bivalent vaccines also recruit new B cells that produce their own antibodies (see ‘Updated vaccines’).
These effects might mean that a bivalent booster provides better protection against Omicron than does a booster dose of the original vaccine. But it’s still unclear how substantial that advantage is in practice.
Some developers, including Pfizer–BioNTech, are also working on combination vaccines to protect people against COVID-19 and other diseases — most commonly influenza. Nearly all are in the early stages of development.
Broadly protective vaccines
Updates to COVID-19 vaccines will always be a step or two behind the evolving virus. Scientists hope to develop ‘broadly protective’ vaccines that can target future SARS-CoV-2 variants — and even related coronaviruses.
The goal of some of these vaccines is to generate an immune response against particular regions of the spike protein that are conserved across SARS-CoV-2 variants and some related coronavirus species, meaning that they tend not to mutate in new variants. One region of interest is the receptor-binding domain (RBD), which binds to the ACE2 receptor protein on human cells and is targeted by some of the body’s most potent infection-blocking antibodies.
At least two teams, at the University of Washington in Seattle and at the California Institute of Technology (Caltech) in Pasadena, are making ‘mosaic’ vaccines: nanoparticles dotted with RBDs from SARS-CoV-2 and coronaviruses from the same family (called sarbecoviruses), such as SARS-CoV and others isolated from bats.
When a B cell recognizes more than one RBD on these mosaic nanoparticles — latching on to conserved regions from multiple virus species — it binds strongly. This, in turn, triggers that B cell to multiply and produce more antibodies (as well as memory B cells to fight future infections). B cells that recognize an RBD from just one viral species bind weakly, and do not generate this response. Researchers hope that using mosaic nanoparticles will result in an enriched pool of antibodies that can recognize multiple RBDs across coronavirus species (see ‘Broader protection?’).
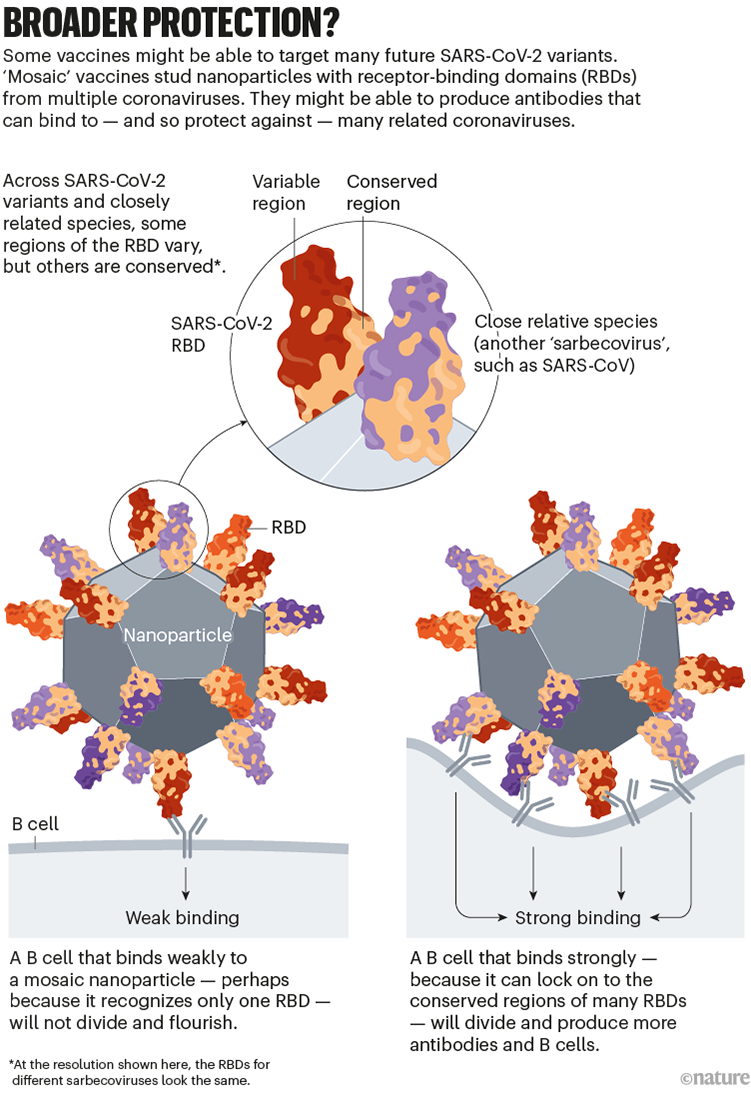
Animal studies suggest that these vaccines do trigger protective responses against diverse sarbecoviruses (see, for example, A. A. Cohen et al. Science 377, eabq0839; 2022). The first clinical trials are set to begin in the next two years.
Going beyond spike
Many first-generation COVID-19 vaccines prompt an immune response only against SARS-CoV-2’s spike protein.
But some next-generation vaccines deliver other viral proteins as well, in the hope of generating a more diverse immune response that safely mimics the protection conferred by infection. This approach could also mitigate the impact of new spike variants (see ‘Targeting other viral proteins’).
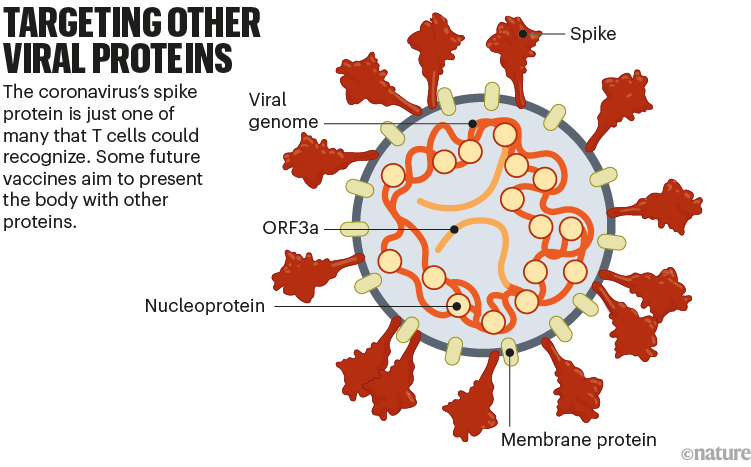
The spike protein is the main target of antibody-making B cells. But T cells that destroy infected cells can recognize many other SARS-CoV-2 proteins. For this reason, vaccines that deliver other proteins could help protect people whose immune systems do not generate strong antibody responses. Such vaccines might also be more resilient to viral evolution, because non-spike proteins tend to vary less between variants.
The US biotechnology company Gritstone is developing one such vaccine: it delivers instructions for several SARS-CoV-2 proteins using mRNA vaccine technology. Meanwhile, Texas biotech company Vaxxinity is developing a protein-based vaccine that would expose the body to multiple antigens. The company says it plans to apply for UK and Australian authorization this year, after a phase III trial showed the vaccine was safe and prompted a strong antibody response when used as a booster.
New platform designs
Another way of categorizing next-generation vaccines is by the method of delivery into the body. Existing vaccines use one of at least four approaches: nucleic-acid vaccines (mostly mRNA) instruct cells to make the SARS-CoV-2 spike protein; inactivated vaccines use versions of the coronavirus itself; protein vaccines are composed of the spike protein or its RBD; and viral-vector vaccines use modified viruses to shuttle instructions for the spike protein into cells. Next-generation vaccines could involve tweaks to these designs or changes to delivery mechanisms that might improve performance.
Self-amplifying RNAs
mRNA vaccines helped to turn the tide of the pandemic, particularly in wealthy countries, where the vast majority of doses have been sold. A twist on this technology might make vaccines cheaper and even more potent, while minimizing side effects.
The vaccines developed by Pfizer–BioNTech and by Moderna (with the US National Institute of Allergy and Infectious Diseases) consist of mRNA instructions for a modified version of spike packaged in a fatty nanoparticle. In an updated version of this technology, self-amplifying RNA (saRNA) vaccines also include instructions for an enzyme that instructs cells to churn out more copies of spike (see ‘Self-amplifying RNA’).
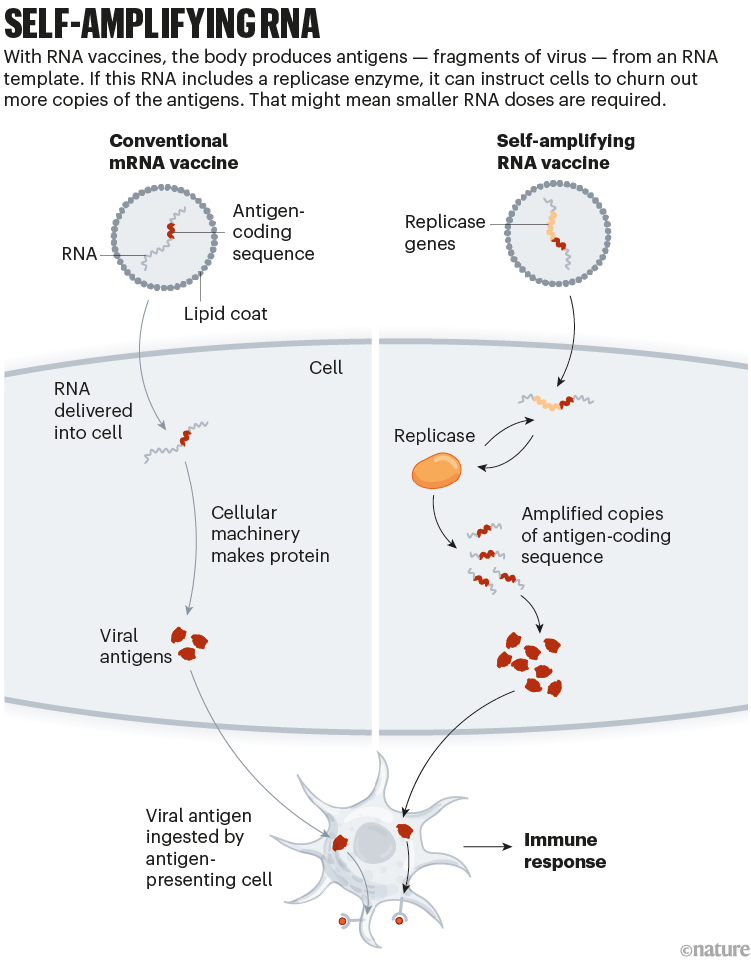
This means that a smaller — and potentially cheaper — dose of saRNA vaccines could achieve the same or even a stronger immune response, compared with conventional mRNA vaccines. A smaller initial dose might also reduce side effects.
One saRNA vaccine, developed by US firm Arcturus Therapeutics, completed a phase III trial in April 2022; the company has now started another phase III trial in Japan that, it says, could lead to an application for authorization there. Gritstone is using saRNA technology to deliver additional SARS-CoV-2 proteins in a candidate T-cell vaccine that has completed a phase I trial.
Proteins on nanoparticles
Several protein-based COVID-19 vaccines have been authorized globally, including one made by US biotech firm Novavax. Their low cost and ease of production makes them appealing; they are usually made of stabilized forms of the entire SARS-CoV-2 spike protein or its RBD.
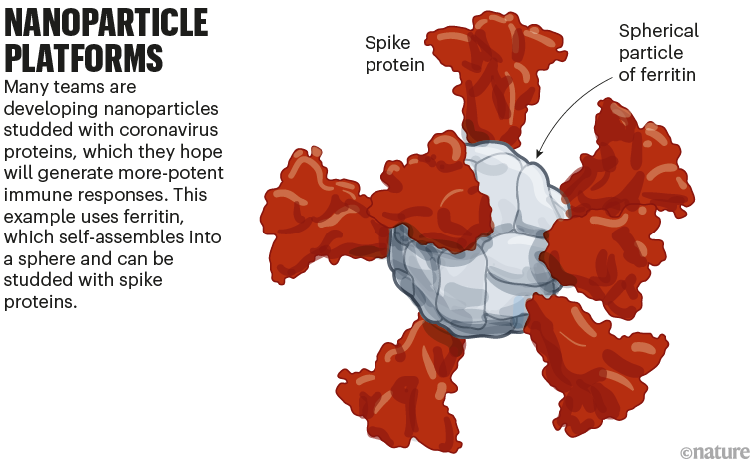
A new class of these vaccines is made of proteins that self-assemble into a soccer-ball-shaped structure, studded with spike or RBD. The repetitive arrangement of the viral molecules, mimicking an actual virus, generates an especially potent immune response.
The ‘mosaic’ vaccines developed by Caltech and the University of Washington (which are studded with RBDs from multiple kinds of coronavirus) are one example of this effort.
Another nanoparticle vaccine has already been approved: in April 2022, South Korean regulators authorized a vaccine, also developed at the University of Washington, containing RBDs from the original version of SARS-CoV-2. A phase III trial showed that the vaccine boosted antibody responses to levels that were several times higher than those generated by the viral-vector vaccine developed by AstraZeneca and the University of Oxford, UK, which uses a chimpanzee adenovirus encoding spike antigens.
However, the South Korean company developing the vaccine, SK biosciences, said in late 2022 that it had paused production amid low demand for the vaccine in South Korea.
A team led by researchers at the US Walter Reed Army Institute of Research in Silver Spring, Maryland, is developing another protein nanoparticle vaccine, using an iron-carrying protein called ferritin. This self-assembles into a spherical particle, and is then studded with the full SARS-CoV-2 spike protein. It is currently being tested in an early-stage trial (see ‘Nanoparticle platforms’).
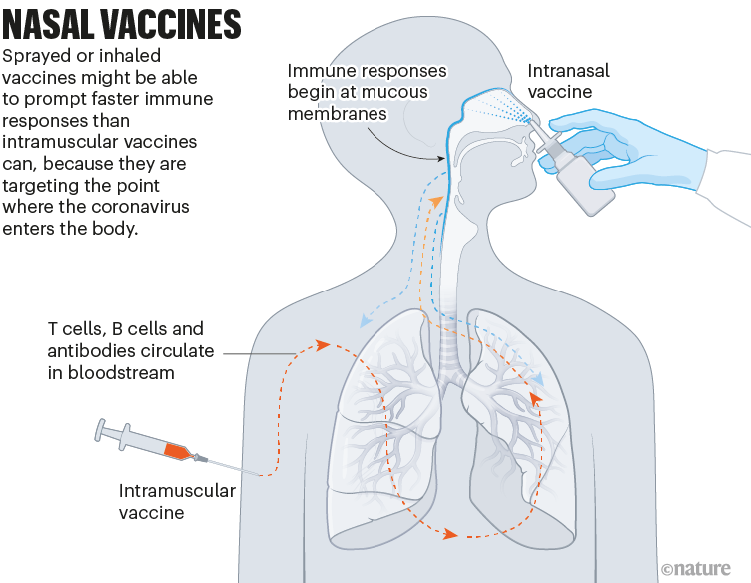
Nasal vaccines
Some COVID-19 vaccines are inhaled as a mist through the nose or mouth, or as nasal drops. By prompting immune responses at the point where SARS-CoV-2 enters the body — in the thin mucous membranes that line the nose and mouth — these vaccines could, in theory, stop the virus before it spreads.
Data from animal studies suggest this might be possible, and at least five nasal vaccines have already been approved for use — two in China and one each in India, Iran and Russia. But there are no data yet on whether these vaccines are better than injections at cutting down infection or transmission of the virus (see ‘Nasal vaccines’).
A key challenge to the development of these and other next-generation COVID-19 vaccines is proving that they offer genuine improvements over existing jabs, says Melanie Saville, executive director of vaccine research and development at the Coalition for Epidemic Preparedness Innovations (CEPI), an Oslo-based foundation that is a leading funder of next-generation COVID-19 vaccines.
Stiff competition
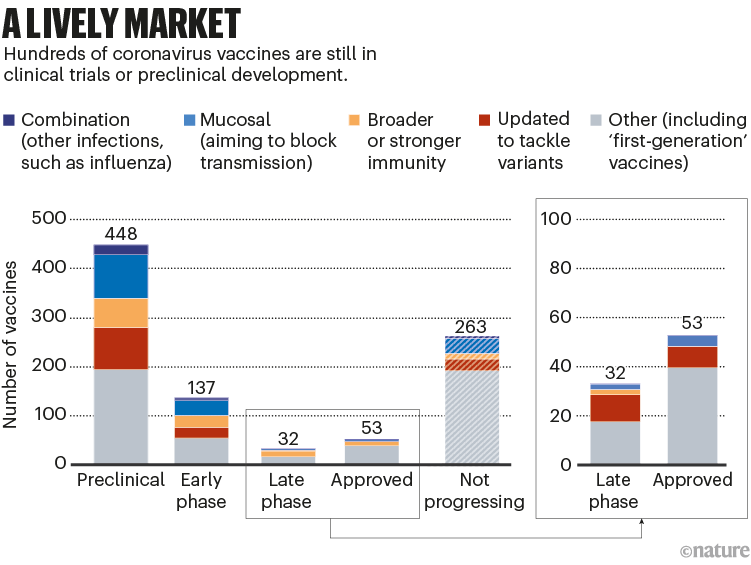
All the next-generation vaccines will have to fight for market share. More than 50 vaccines have already been approved, and there are hundreds in early- and late-stage clinical trials; hundreds more have been abandoned (see ‘A lively market’).
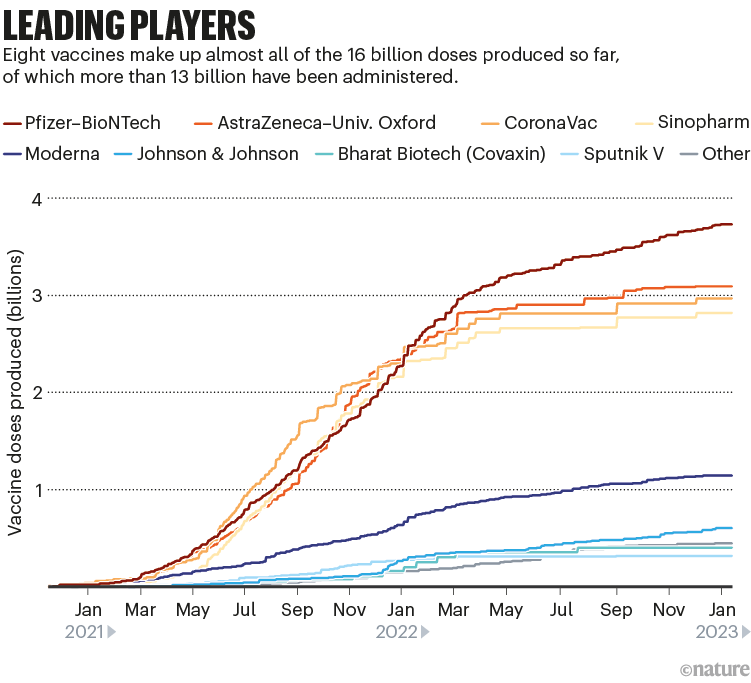
Among the approved vaccines, just a few dominate the doses that have been administered (see ‘Leading players’).
Despite the flurry of research, current mRNA jabs such as the Moderna and Pfizer–BioNTech ones are likely to hold sway, says Matt Linley, analytics director at Airfinity, a life-sciences information firm in London. The fast development of bivalent vaccines that included an Omicron component showed that these vaccines could be adapted quickly. If another update is needed, “mRNA vaccinations would be market leaders in being able to react quickly”, says Linley.
COVID-19 is no longer seen as the all-encompassing emergency it once was, and so developers and regulators will move more slowly compared with the breakneck pace of the first-generation vaccines, adds Saville. “We shouldn’t rush these through, because these need to be the types of vaccine that will be durable in the long term for COVID-19.”
But even if work on new vaccine technologies doesn’t directly pay off against COVID-19, it could still support efforts to combat other diseases, Saville says, such as CEPI’s work on a ‘vaccine library’ for different virus families to improve preparation for future threats.
Nature 614, 22-25 (2023)
doi: https://doi.org/10.1038/d41586-023-00220-z
“热闹的市场”——下一代冠状病毒疫苗
为了应对COVID-19疫情,迄今数十亿人接种了SARS-CoV-2冠状病毒疫苗,这挽救了超2000多万人的生命。但病毒变体可以逃避初始版疫苗提供的一些免疫力。因此,世界各地开发人员正在研制多种“下一代”COVID-19疫苗,他们不仅对初始版疫苗进行了更新,还使用了新的技术和平台。
这些疫苗是多样化的,但首要目标是提供持久的保护以抵御病毒变化。它们中有些可以抵御更广泛的冠状病毒,包括尚未出现的冠状病毒。其他疫苗可能以更低的剂量提供更强大的免疫力,或者可能更好地预防病毒感染或传播。
2月1日,发表在Nature杂志上的一篇文章,通过10张图展望了“下一代”COVID-19疫苗。
为什么需要更多疫苗?
一个词:进化。最先获批的COVID-19疫苗接受了SARS-CoV-2(自病毒首次鉴定以来没有太大变化)的保护力测试。这些疫苗有不同的类型,有些是由信使RNA(mRNA)组成,有些是灭活冠状病毒本身或其某些蛋白质,但所有疫苗都是通过将身体暴露于抗原(病毒的一部分)在不致病的情况下激发免疫反应而起作用。
广义地说,这种免疫反应来自B细胞和T细胞。B细胞产生的抗体可以阻止SARS-CoV-2感染细胞,T细胞可以破坏感染细胞,并支持其他免疫反应。
即使在最初的抗体水平下降后,接种疫苗也会产生一批“记忆细胞”,以延长免疫力。在随后的感染中,记忆B细胞开始增殖并分化为产生更多抗体的细胞。
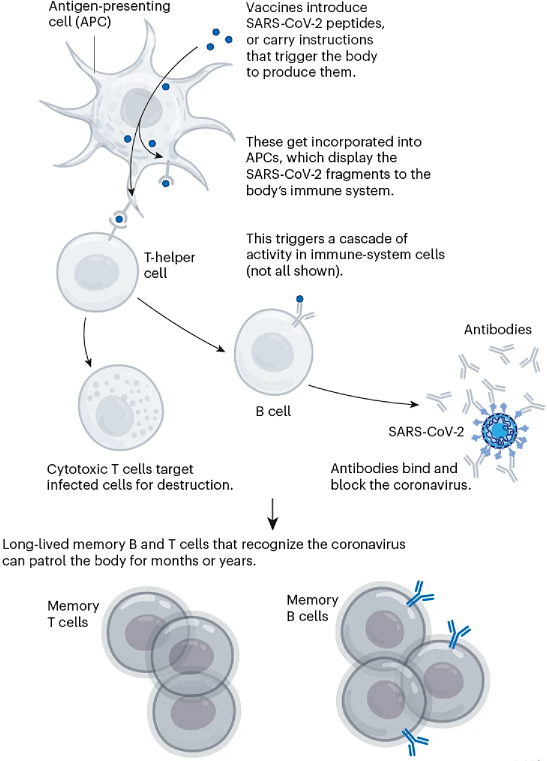
图1 | 冠状病毒疫苗如何预防SARS-COV-2。(来源:Nik Spencer/Nature)
尽管这些疫苗对预防COVID-19重症提供了持久的保护,但对病毒感染的保护在几个月内就会减弱。SARS-CoV-2的变体,如奥密克戎,已经进化出突变,使其能够逃脱部分这种免疫。例如,初始版疫苗提供的记忆应答产生的抗体不易与奥密克戎结合,使其对感染的保护力降低。
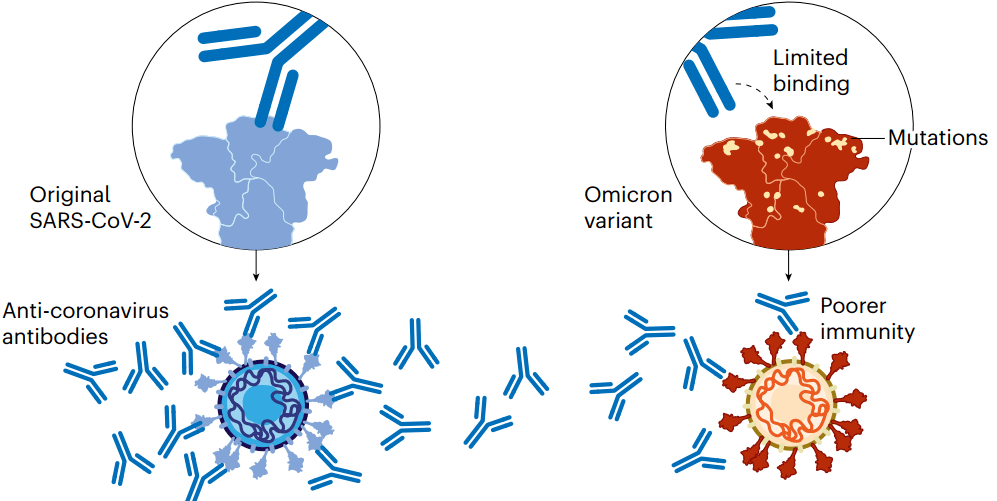 图2 | 冠状病毒变体逃避免疫系统。(来源:Nik Spencer/Nature)
图2 | 冠状病毒变体逃避免疫系统。(来源:Nik Spencer/Nature)
已经推出的第二代疫苗提高了对奥密克戎变异株的免疫应答。当然,鉴于病毒的不断进化,研究人员很可能会对疫苗进行进一步针对变体的更新,因此,他们正在采取多种方法开发新疫苗。
原版疫苗升级
为了应对SARS-CoV-2变体,疫苗开发商Pfizer-BioNTech和Moderna去年推出了最新的二价mRNA疫苗。它们编码原始病毒和奥密克戎的Spike蛋白(SARS-CoV-2用其与宿主细胞结合)分子。
二价疫苗有几种作用:1)与其他COVID-19疫苗强化针一样,它们刺激了先前疫苗已经建立的记忆B细胞;2)这种细胞应答的一部分产生了能够识别奥密克戎的抗体,随着时间的推移,它们的效力也会增强:当出现奥密克戎的Spike蛋白时,记忆B细胞会经历突变和选择的进化“训练”过程,产生一批成熟B细胞,它们编码与奥密克戎Spike蛋白结合更紧密的抗体。3)最后,二价疫苗的奥密克戎成分也会招募产生自身抗体的新的B细胞。
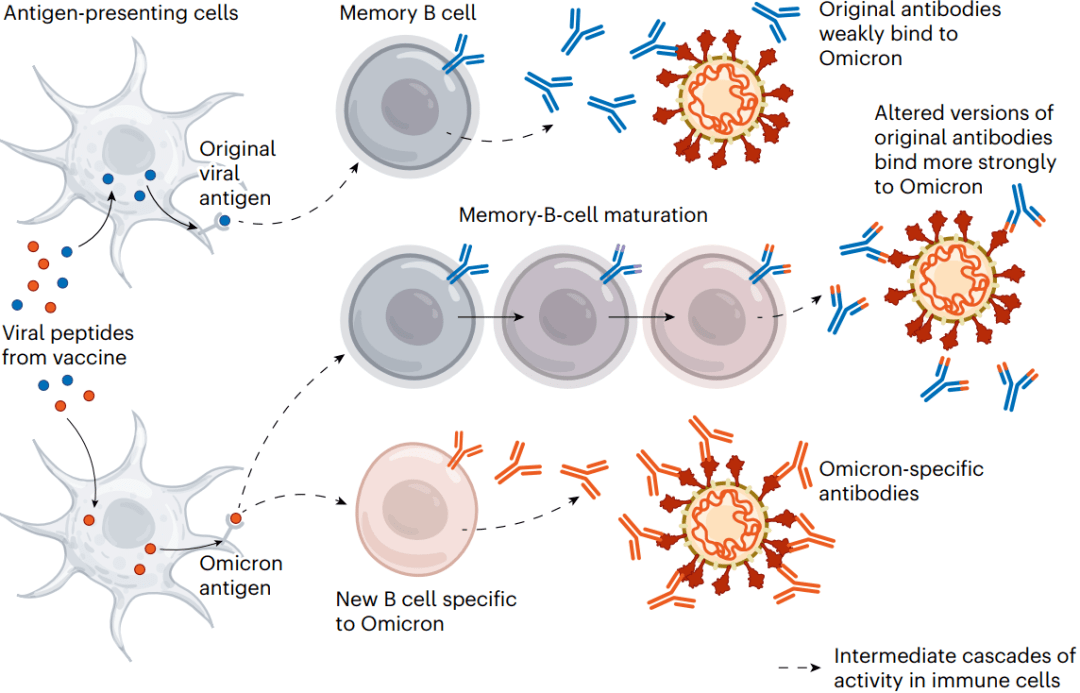 图3 | 更新的疫苗。二价疫苗同时含有原始病毒及其变体,如奥密克戎的一部分,以尝试跟上不断进化的病毒。
图3 | 更新的疫苗。二价疫苗同时含有原始病毒及其变体,如奥密克戎的一部分,以尝试跟上不断进化的病毒。
这些影响可能意味着,相比于原始疫苗加强针,二价疫苗加强针能提供更好的对抗奥密克戎的保护力。但目前尚不清楚这种优势在实际应用中有多大。
此外,包括Pfizer-BioNTech在内的一些开发商也在研究联合疫苗,以保护人们免受COVID-19和其他疾病(最常见的是流感)的侵害。这些几乎都处于开发的早期阶段。
广泛保护性疫苗
COVID-19疫苗的更新将始终落后于病毒的进化。因此,科学家们希望开发出“广泛保护性”疫苗,以针对未来的SARS-CoV-2变体,甚至相关冠状病毒。
其中一些疫苗的目标是针对SARS-CoV-2变体和一些相关冠状病毒株中保守的Spike蛋白特定区域(它们倾向于不在新变体中发生突变)产生免疫反应。一个有吸引力的区域是受体结合域(RBD),它与人体细胞上的ACE2受体蛋白结合,并被人体最有效的感染阻断抗体靶向。
华盛顿大学和加州理工学院至少有两个团队正在制作“马赛克”疫苗,一种“点缀”着SARS-CoV-2和来自同一家族的冠状病毒(称为sarbecoviruses,如SARS-CoV和其他分离自蝙蝠的病毒)RBD的纳米颗粒 。
当一个B细胞识别这些镶嵌在纳米颗粒上的多个RBD时,它会与多种病毒保守区域紧密结合。这反过来触发B细胞增殖并产生更多抗体(以及记忆B细胞以对抗未来的感染)。只识别一种病毒的RBD的B细胞结合较弱,不会产生这种反应。研究人员希望,通过使用“马赛克”纳米颗粒产生一个丰富的抗体库,可以识别冠状病毒种类中的多种RBD。
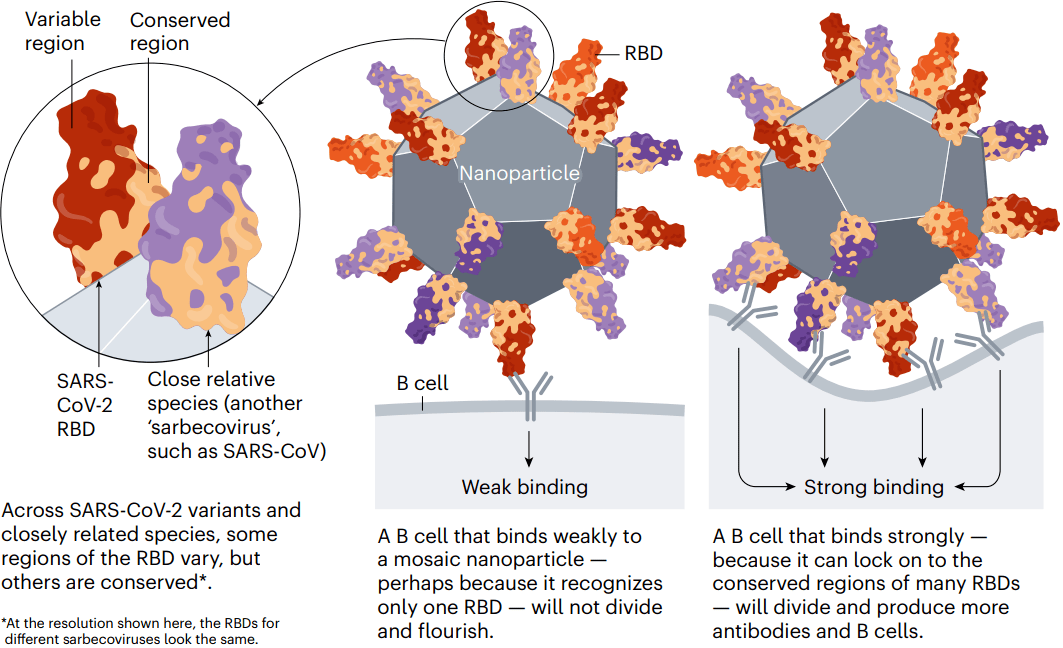 图4 | 更广泛的保护。
图4 | 更广泛的保护。
动物研究表明,这些疫苗确实会引发针对不同sarbecoviruses的保护性反应。首批临床试验将在未来两年内开始。
超越Spike蛋白
许多第一代COVID-19疫苗只对SARS-CoV-2的Spike蛋白产生免疫反应。但一些下一代疫苗还能递送其他病毒蛋白,希望能产生更多样的免疫反应,安全模拟感染所带来的保护。这种方法还可以减轻新的Spike变体的影响。
图5 | 靶向其他病毒蛋白。冠状病毒的Spike蛋白只是T细胞能够识别的众多蛋白之一。一些未来的疫苗旨在为人体提供其他蛋
Spike蛋白是产生抗体的B细胞的主要靶点。但破坏感染细胞的T细胞可以识别许多其他SARS-CoV-2蛋白。因此,递送其他蛋白质的疫苗可以帮助保护免疫系统不能产生强烈抗体反应的人。这类疫苗可能对病毒进化更有抵抗力,因为非Spike蛋白在变体之间的差异较小。
美国生物技术公司Gritstone正在开发一种这样的疫苗:它使用mRNA疫苗技术为几种SARS-CoV-2蛋白递送指令。与此同时,另一家美国生物技术公司Vaxxinity正在开发一种可以使人体暴露于多种抗原的基于蛋白质的疫苗,计划今年向英国和澳大利亚管理机构递交申请。
新平台设计
对下一代疫苗进行分类的另一个维度是向体内递送的方法。现有疫苗使用至少4种方法中的一种:核酸疫苗(主要是mRNA)指导细胞制造SARS-CoV-2 Spike蛋白;灭活疫苗使用失活的冠状病毒本身;蛋白质疫苗由Spike蛋白或其RBD组成;病毒载体疫苗使用修饰的病毒将Spike蛋白的指令递送到细胞中。下一代疫苗可能需要对这些设计进行调整,或改变可能提高性能的递送机制。
自扩增RNA
Pfizer-BioNTech和Moderna(与美国国家过敏和传染病研究所合作)开发的疫苗包含了包装在脂肪纳米颗粒中的改良型Spike蛋白mRNA指令。在这项技术的更新版本中,自扩增RNA(saRNA)疫苗还包括一种酶的指令,该酶指示细胞产生更多的Spike蛋白拷贝。
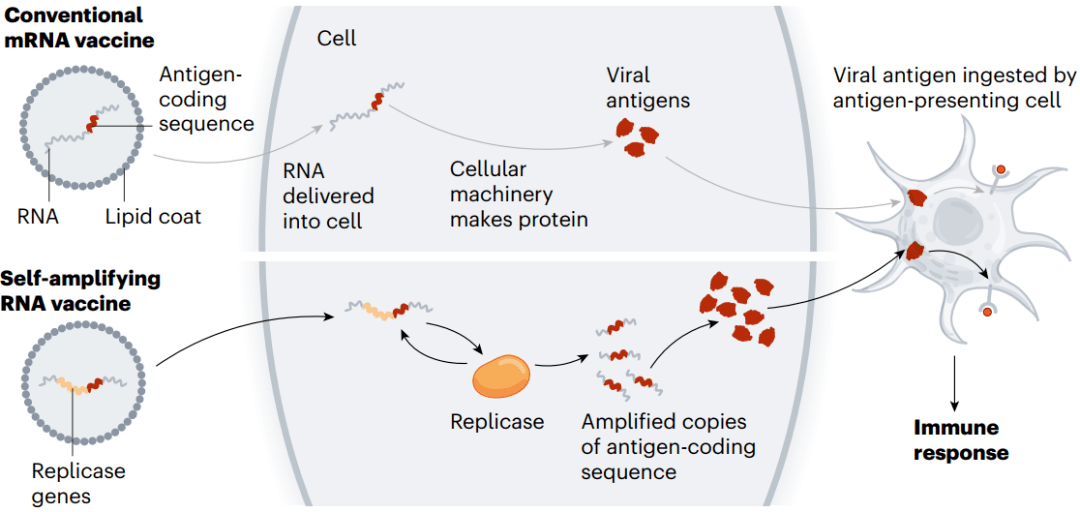 图6 | 自扩增RNA。(来源:Nik Spencer/Nature)
图6 | 自扩增RNA。(来源:Nik Spencer/Nature)
这意味着,与常规mRNA疫苗相比,更小剂量且可能更便宜的saRNA疫苗可以实现相同甚至更强的免疫应答。较小的初始剂量也可能减少副作用。
一种由美国Arcturus Therapeutics公司开发的saRNA疫苗于2022年4月完成了III期试验;该公司目前已开始在日本进行另一项III期试验。
纳米颗粒上的蛋白质
一些基于蛋白质的COVID-19疫苗已在全球获得授权,其中包括一种由美国生物技术公司Novavax生产的疫苗。它们的低成本和易生产性使它们具有吸引力;它们通常由整个SARS-CoV-2 spike蛋白或其RBD的稳定形式制成。
一类新的疫苗是由可以自行组装成一个足球状结构的蛋白质制成的。这些蛋白质镶嵌有Spike蛋白或RBD,病毒分子的重复排列模仿真实病毒,能产生特别强大的免疫反应。上文提及的“马赛克”疫苗就是一个例子。
此外,另一种纳米颗粒疫苗已经获得批准:2022年4月,韩国监管机构批准了一种同样由华盛顿大学研发的疫苗,该疫苗含有SARS-CoV-2原始版本的RBD。一项III期试验表明,该疫苗将抗体反应提高到比阿斯利康和英国牛津大学开发的病毒载体疫苗(使用一种编码Spike蛋白抗原的黑猩猩腺病毒)高出几倍的水平。
美国Walter Reed陆军研究所的研究团队正使用一种携带铁的蛋白质ferritin,开发另一种蛋白质纳米颗粒疫苗。其可自组装成球形颗粒,然后镶嵌上完整的SARS-CoV-2 Spike蛋白。目前正在进行早期试验。
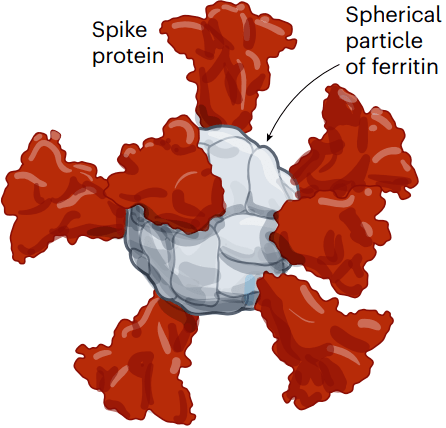
图7 | 纳米颗粒平台。
许多研究团队正在开发镶嵌有冠状病毒蛋白的纳米颗粒,希望其能产生更强大的免疫反应。这个例子使用的是铁蛋白,它可以自行组装成一个球体,并可以镶嵌spike蛋白。(来源:Nik Spencer/Nature。改编自斯坦福大学https://go.nature.com/3WV2FB6 和WRAIR https://go.nature.com/3JTX3XU)
鼻腔疫苗
一些COVID-19疫苗通过鼻或口吸入或作为滴鼻剂给药。理论上,这些疫苗通过在SARS-CoV-2进入人体的部位(口鼻薄黏膜)激发免疫反应,可以在病毒传播之前对其进行阻断。
来自动物研究的数据表明这是有可能的,且至少有5种鼻用疫苗已经被批准使用,其中两种在中国,印度、伊朗和俄罗斯各一款。但目前还没有数据表明这些疫苗在减少病毒感染或传播方面是否优于注射。
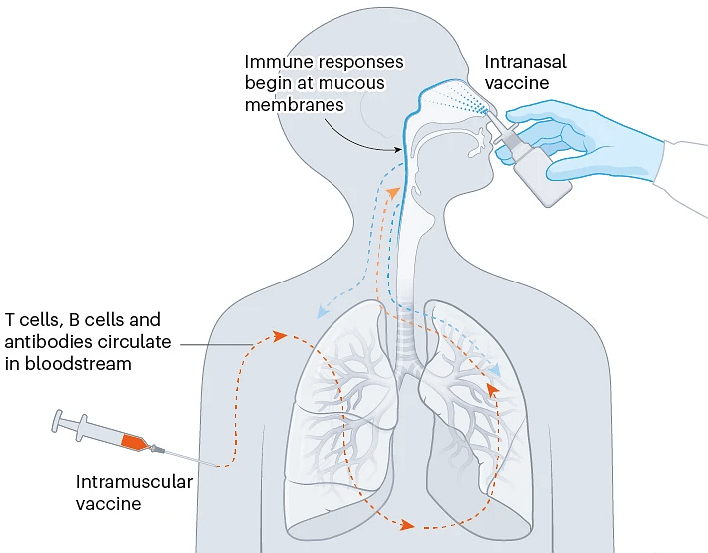
图8 | 鼻腔疫苗。
喷洒或吸入疫苗可能能够比肌肉注射疫苗更快地促进免疫反应,因为它们针对的是冠状病毒进入人体的部位。(来源:Nik Spencer/Nature)
来自流行病防范创新联盟(CEPI,一家基金会,是下一代COVID-19疫苗的主要资助者)的Melanie Saville表示,开发这些和其他新一代COVID-19疫苗的一个关键挑战是证明它们比现有疫苗有了真正的改进。
激烈的竞争
所有下一代疫苗都必须争夺市场份额。目前已有50多种疫苗获批,有数百种疫苗处于早期和晚期临床试验阶段,还有数百种被放弃。
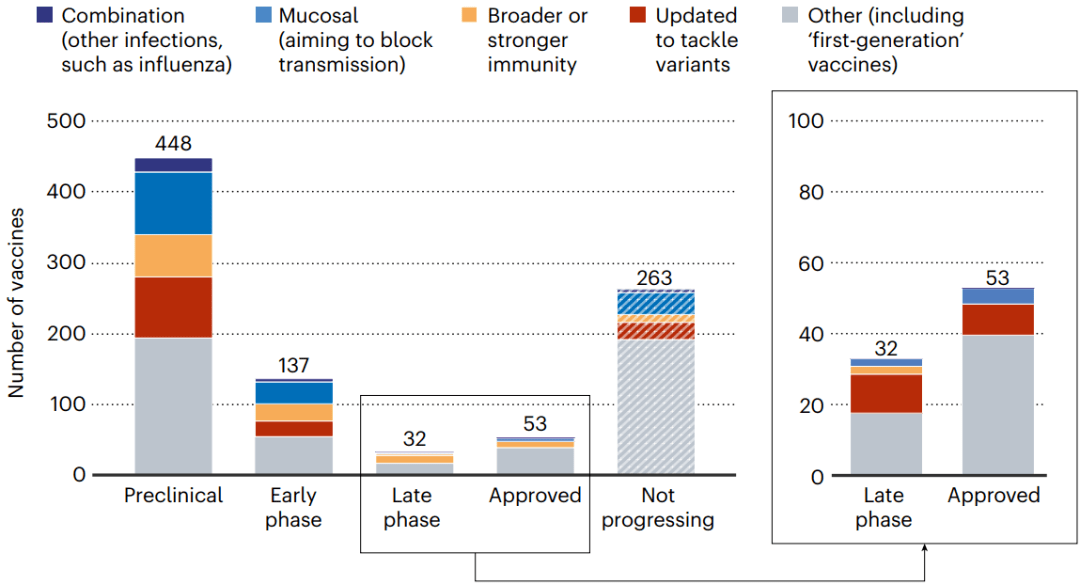 图9 | 所有这些新一代疫苗都将争夺市场份额。(来源:Nik Spencer/Nature. 摘自Airfinity)
图9 | 所有这些新一代疫苗都将争夺市场份额。(来源:Nik Spencer/Nature. 摘自Airfinity)
在已批准的疫苗中,只有少数疫苗占接种剂量的主导地位。
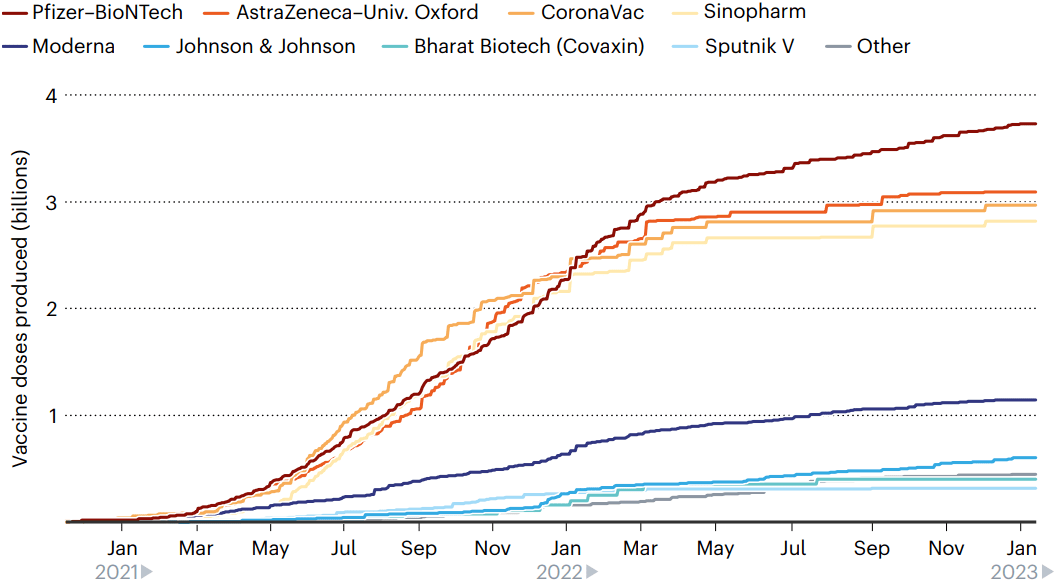 图10 | 领先玩家。
图10 | 领先玩家。
目前生产的160亿剂疫苗中,有8种疫苗几乎占了全部,其中130多亿剂已接种。(来源:Nik Spencer/Nature,摘自Airfinity)
尽管全球有大量研究进行,但目前的mRNA疫苗(如Moderna 和 Pfizer–BioNTech的)可能会占据主导地位。包含奥密克戎成分的二价疫苗的快速开发表明,这些疫苗可以快速调整。如果出现新的病毒变体,需要再度更新,mRNA疫苗将成为市场领导者,因为它们能够快速做出反应。
COVID-19不再被视为曾经需要全面警戒的情况,因此与第一代疫苗开发的惊人速度相比,现在开发人员和监管机构的行动将缓慢一些。我们不应急于完成这些,因为这些疫苗需要是对COVID-19持久有效的。即便新疫苗技术的研究不能直接对抗COVID-19,它们仍可以支持抗击其他疾病。例如CEPI为不同的病毒家族建立“疫苗库”,以改进对未来威胁的准备工作。